262536
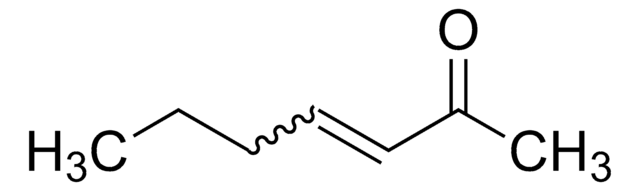
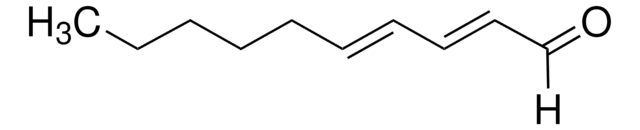
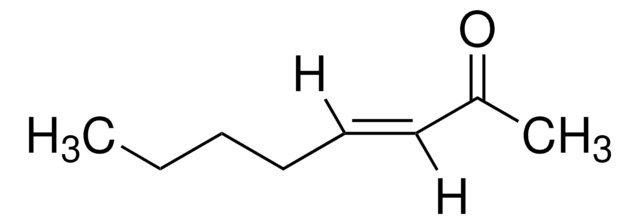
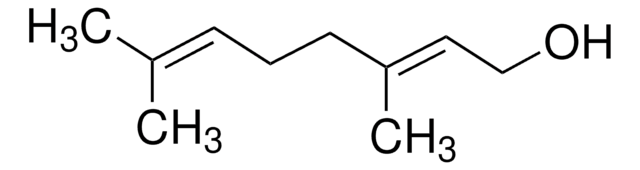
trans-3-Nonen-2-one
95%
Synonym(s):
(3E)-3-Nonen-2-one, (3E)-Non-3-en-2-one, (E)-3-Nonen-2-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)4CH=CHCOCH3
CAS Number:
Molecular Weight:
140.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.449 (lit.)
bp
85 °C/12 mmHg (lit.)
density
0.848 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
[H]\C(CCCCC)=C(\[H])C(C)=O
InChI
1S/C9H16O/c1-3-4-5-6-7-8-9(2)10/h7-8H,3-6H2,1-2H3/b8-7+
InChI key
HDKLIZDXVUCLHQ-BQYQJAHWSA-N
Application
trans-3-Nonen-2-one has been used:
- as substrate to investigate steady-state kinetics of NADPH oxidation
- in the preparation of azido quinolone
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hajoong Lee et al.
The Journal of organic chemistry, 75(5), 1756-1759 (2010-02-10)
We describe the assembly of a 960-member library of tricyclic 2,3-dihydro-4-quinolones using a combination of solution-phase high-throughput organic synthesis and parallel chromatographic purification. The library was produced with high efficiency and complete chemo- and diastereoselectivity by diversification of an azide-bearing
Ryan A Dick et al.
The Journal of biological chemistry, 279(17), 17269-17277 (2004-02-18)
NADPH-dependent alkenal/one oxidoreductase (AOR) from the rat is a phase 2/antioxidative enzyme that is known to catalyze the reduction of the carbon-carbon double bond of alpha,beta-unsaturated aldehydes and ketones. It is also known for its leukotriene B(4) 12-hydroxydehydrogenase activity. In
Emiliano Ventura et al.
Journal of chromatography. A, 1600, 183-196 (2019-05-06)
A semi-quantitative method was developed to monitor the misuse of 15 SARM compounds belonging to nine different families, in urine matrices from a range of species (equine, canine, human, bovine and murine). SARM residues were extracted from urine (200 μL) with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service