252727
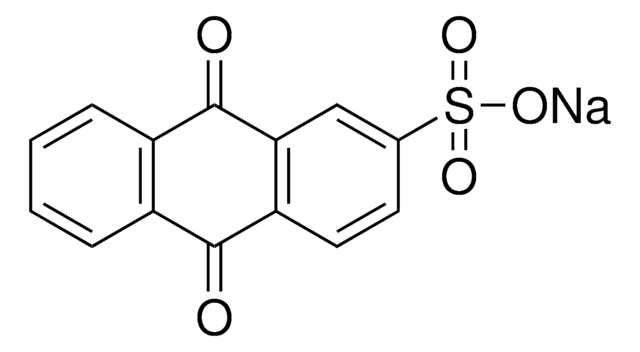
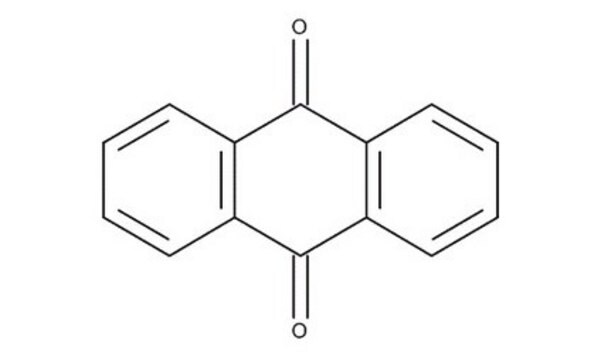
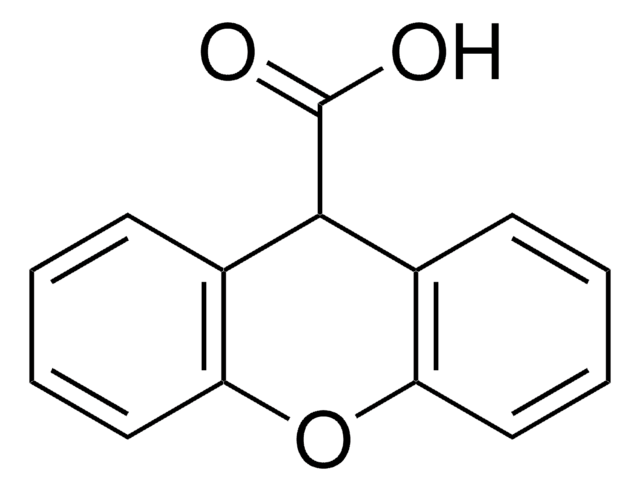
Anthraquinone-2-carboxylic acid
98%
Synonym(s):
9,10-Dihydro-9,10-dioxo-2-anthracenecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H8O4
CAS Number:
Molecular Weight:
252.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
287-289 °C (lit.)
functional group
carboxylic acid
ketone
SMILES string
OC(=O)c1ccc2C(=O)c3ccccc3C(=O)c2c1
InChI
1S/C15H8O4/c16-13-9-3-1-2-4-10(9)14(17)12-7-8(15(18)19)5-6-11(12)13/h1-7H,(H,18,19)
InChI key
ASDLSKCKYGVMAI-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Y Tsai et al.
Journal of pharmaceutical sciences, 82(12), 1250-1254 (1993-12-01)
The physicochemical properties of 9,10-anthraquinone-2-carboxylic acid (AQCA) were investigated by thermal analysis, powder X-ray diffraction pattern, solubility, and partition coefficient. The chemical structure of AQCA was confirmed by the data from UV, Fourier transform IR (FTIR), and NMR analyses. Solubility
Coupled Proton and Electron Transfer: Adsorbed Anthraquinone-2-carboxylic Acid Monolayers.
Forster RJ.
Journal of the Electrochemical Society, 144(4), 1165-1173 (1997)
Yiwen Zhu et al.
Materials (Basel, Switzerland), 13(16) (2020-08-17)
Antimicrobial and antiviral materials have attracted significant interest in recent years due to increasing occurrences of nosocomial infections and pathogenic microbial contamination. One method to address this is the combination of photoactive compounds that can produce reactive oxygen species (ROS)
A Bielawska et al.
Folia histochemica et cytobiologica, 39 Suppl 2, 207-208 (2002-02-01)
Although prolidase [E.C.3.4.13.9] is found in normal cells, substantially increased levels are found in some neoplastic tissues. Prolidase evokes the ability to hydrolyse the imido-bond of various low molecular weight compounds coupled to L-proline. The synthesis of three proline analogues
A Bielawska et al.
Roczniki Akademii Medycznej w Bialymstoku (1995), 43, 201-209 (1999-02-11)
The feasibility to targeting prolidase as an antineoplastic prodrug--converting enzyme has been examined. The synthesis of proline analogue of anthraquinone-2-carboxylic acid (potential antineoplastic agent) conjugated through imido-bond (potential target for prolidase action) has been performed. The product was found to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service