232270
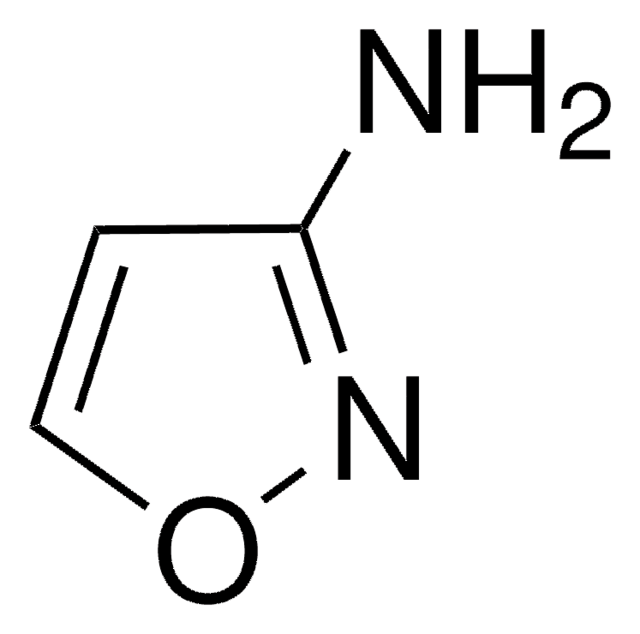
3-Amino-5-methylisoxazole
≥97%
Synonym(s):
5-Methyl-3-isoxazolamine, NSC 159134
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6N2O
CAS Number:
Molecular Weight:
98.10
Beilstein:
108110
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
solid
mp
59-61 °C (lit.)
SMILES string
Cc1cc(N)no1
InChI
1S/C4H6N2O/c1-3-2-4(5)6-7-3/h2H,1H3,(H2,5,6)
InChI key
FKPXGNGUVSHWQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Amino-5-methylisoxazole is the major intermediate formed during sulfamethoxazole biodegradation by Pseudomonas psychrophila strain HA-4. It is the intermediate formed during the photocatalytic degradation of sulfamethoxazole (SMX).
Application
3-Amino-5-methylisoxazole was used in synthesis of:
- naphtho[1,2-e][1,3]oxazines
- series of 1-aryl-4-methyl-3,6-bis-(5-methylisoxazol-3-yl)-2-thioxo-2,3,6,10b-tetrahydro-1H-pyrimido[5,4-c]quinolin-5-ones, having potential mosquito larvicidal activity
- hydroxylamines of sulfadiazine and sulfamethoxazole
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lu Wang et al.
Water research, 88, 322-328 (2015-10-30)
Sulphamethoxazole (SMX) is extensively used in humans and livestock, but its appearance in natural water raises environmental concerns. This study demonstrated that SMX and its degradation product, 3-amino-5-methylisoxazole (3A5MI), could be effectively degraded in microbial fuel cell (MFC) reactors. Approximately
Shiyuan Ding et al.
Journal of hazardous materials, 262, 812-818 (2013-10-22)
Photocatalytic degradation of sulfamethoxazole (SMX) was investigated using Bi2O3/Bi2O2CO3/Sr6Bi2O9 (BSO) photocatalyst under visible light (>420 nm) irradiation. The photochemical degradation of SMX followed pseudo-first-order kinetics. The reaction kinetics was determined as a function of initial SMX concentrations (5-20 mg L(-1))
Benchao Jiang et al.
Applied microbiology and biotechnology, 98(10), 4671-4681 (2014-02-14)
Sulfamethoxazole is a common antibiotic that is frequently detected in wastewater and surface water. This study investigated the biodegradation and metabolic pathway of sulfamethoxazole by Pseudomonas psychrophila HA-4, a cold-adapted bacterium. Strain HA-4, which uses sulfamethoxazole as its sole source
Mehdi Shafiee et al.
Molecular diversity, 16(4), 727-735 (2012-10-24)
An expeditious, straightforward and efficient synthesis of diversely naphtho[1,2-e][1,3]oxazines via one-pot condensation reaction of β- naphthol, 3-amino-5-methylisoxazole and arylaldehydes catalyzed by bismuth(III) trifluoromethanesulfonate is described. The reaction preferentially afforded 1,3-trans oxazines.
Bianca M Souza et al.
Environmental science and pollution research international, 24(7), 6195-6204 (2015-11-12)
The present study aims to assess the removal of 3-amino-5-methylisoxazole (AMI), a recalcitrant by-product resulting from the biological breakdown of some pharmaceuticals, applying a solar photo-Fenton process assisted by ferrioxalate complexes (SPFF) (Fe
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service