218456
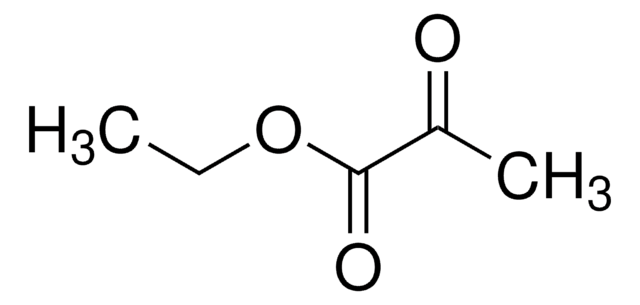
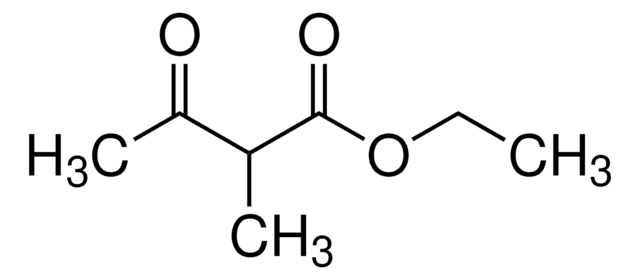
Ethyl 3-methyl-2-oxobutyrate
97%
Synonym(s):
Ethyl dimethylpyruvate, Ketovaline ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
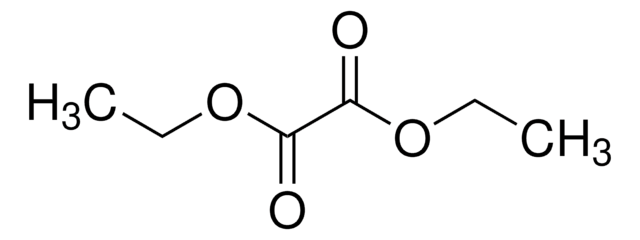
(CH3)2CHCOCOOCH2CH3
CAS Number:
Molecular Weight:
144.17
Beilstein:
1756668
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.410 (lit.)
bp
62 °C/11 mmHg (lit.)
density
0.989 g/mL at 25 °C (lit.)
functional group
ester
ketone
storage temp.
2-8°C
SMILES string
CCOC(=O)C(=O)C(C)C
InChI
1S/C7H12O3/c1-4-10-7(9)6(8)5(2)3/h5H,4H2,1-3H3
InChI key
CKTYYUQUWFEUCO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl 3-methyl-2-oxobutyrate reacts with a variety of allyl halides in the presence of indium to afford hydroxy unsaturated carbonyl compounds. Conformational behaviour of ethyl 3-methyl-2-oxobutyrate was investigated using solution FTIR in combination with ab initio calculations.
Application
Ethyl 3-methyl-2-oxobutyrate was used in the synthesis of (2SR,4SR)-5(S)-(N-Boc)-amino-6-cyclohexyl-4-hydoxy-2-isopropyl hexanoic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
109.4 °F - closed cup
Flash Point(C)
43 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A convenient allylation of 1, n-dicarbonyl compounds using organoindium reagents.
Lee PH, et al.
Bull. Korean Chem. Soc., 22(12), 1380-1384 (2001)
Conformational isomerism of a-ketoesters. A FTIR and ab initio study.
Ferri D, et al.
J. Chem. Soc. Perkin Trans. II, 2, 221-227 (2000)
The synthesis of (2S, 4S, 5S)-5-(N-BOC)-amino-6-cyclohexyl-4-hydroxy-2-isopropyl-hexanoic acid lactone, an hydroxyethylene dipeptide isostere precusor.
Chakravarty PK, et al.
Tetrahedron Letters, 30(4), 415-418 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service