191213
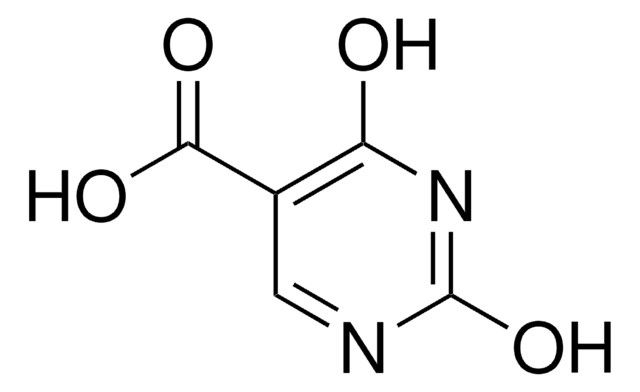
5-Aminoorotic acid
99%
Synonym(s):
5-Amino-2,6-dioxo-1,2,3,6-tetrahydro-4-pyrimidinecarboxylic acid, 5-Amino-6-carboxy-2,4-dihydroxypyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H5N3O4
CAS Number:
Molecular Weight:
171.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
>300 °C (lit.)
functional group
carboxylic acid
SMILES string
NC1=C(NC(=O)NC1=O)C(O)=O
InChI
1S/C5H5N3O4/c6-1-2(4(10)11)7-5(12)8-3(1)9/h6H2,(H,10,11)(H2,7,8,9,12)
InChI key
HWCXJKLFOSBVLH-UHFFFAOYSA-N
General description
5-Aminoorotic acid forms complexes with cerium (III), lanthanum (III) and neodymium (III). It reacts with dianhydride of ethylenedinitrilotetraacetic acid in dry DMF or DMSO to yield functionalized dicarboxamide derivatives.
Application
5-Aminoorotic acid was used as an internal standard during the electrochemical determination of orotic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Irena Kostova et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 4(4), 371-378 (2008-08-05)
The cerium (III), lanthanum (III) and neodymium (III) complexes of 5-aminoorotic acid were synthesized and their structures were determined by means of theoretical study, spectral and physicochemical analysis. Significant differences in the spectra of the complexes were observed as compared
Synthesis and characterisation of chelating polycarboxylate ligands capable of forming intermolecular, complementary triple hydrogen bonds.
Georgopoulou AS, et al.
J. Chem. Soc., Dalton Trans., 11, 1869-1878 (1998)
Tommaso R I Cataldi et al.
Journal of chromatography. A, 1107(1-2), 130-138 (2006-01-13)
The application of activated pulsed amperometric detection (APAD) for the determination of orotic acid (OrA) in real samples at a gold working electrode in alkaline solutions, in combination with anion-exchange chromatography, is reported. Such an activated potential waveform was designed
Rony Panarsky et al.
Cancer & metabolism, 8, 7-7 (2020-08-11)
The loss-of-function mutation of fumarate hydratase (FH) is a driver of hereditary leiomyomatosis and renal cell carcinoma (HLRCC). Fumarate accumulation results in activation of stress-related mechanisms leading to upregulation of cell survival-related genes. To better understand how cells compensate for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service