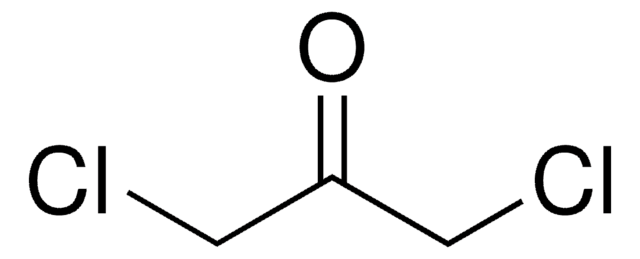
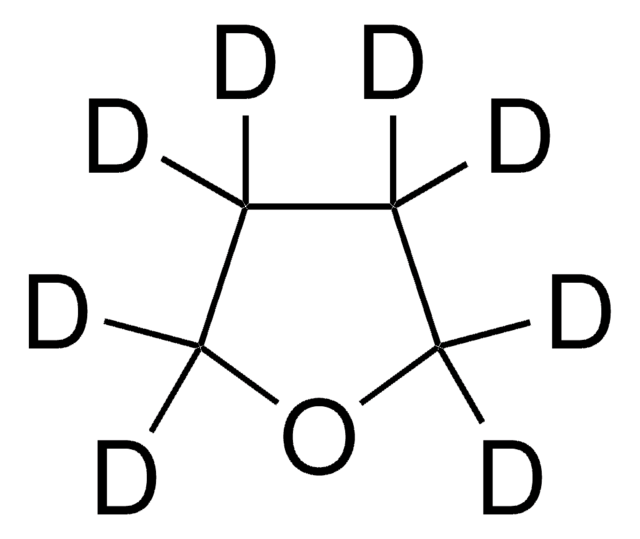
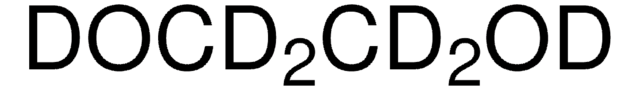186406
1,4-Dioxane-d8
≥99 atom % D
Synonym(s):
p-Dioxane-d8, Octadeuterodioxane
About This Item
Recommended Products
isotopic purity
≥99 atom % D
Assay
≥99% (CP)
form
liquid
technique(s)
NMR: suitable
refractive index
n20/D 1.4198 (lit.)
bp
99 °C (lit.)
mp
12 °C (lit.)
density
1.129 g/mL at 25 °C (lit.)
mass shift
M+8
SMILES string
[2H]C1([2H])OC([2H])([2H])C([2H])([2H])OC1([2H])[2H]
InChI
1S/C4H8O2/c1-2-6-4-3-5-1/h1-4H2/i1D2,2D2,3D2,4D2
InChI key
RYHBNJHYFVUHQT-SVYQBANQSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Neutron Scattering of Residual Hydrogen in 1,4-Dioxane-d8 Liquid: Understanding Measurements with Molecular Dynamics Simulations: This study utilizes 1,4-Dioxane-d8 to enhance neutron scattering measurements, providing crucial data for researchers in material science and analytical chemistry on solvent behavior and molecular interactions (de Almeida et al., 2016).
- Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06: This research highlights the degradation pathways of 1,4-Dioxane-d8, contributing to environmental sciences by offering insights into bioremediation techniques that mitigate solvent impact (Kim et al., 2009).
- Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus: Explores the fungal degradation of cyclic ethers, including 1,4-Dioxane-d8, underscoring its significance in environmental cleanup and testing standards for academic and pharmaceutical research on organic pollutant breakdown (Nakamiya et al., 2005).
Recommended products
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
41.0 °F - closed cup
Flash Point(C)
5 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Use this reference table to find the coupling values and chemical shifts of our NMR (deuterated) solvents. Melting and boiling points, molecular weight, density, and CAS number are also listed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service