183431
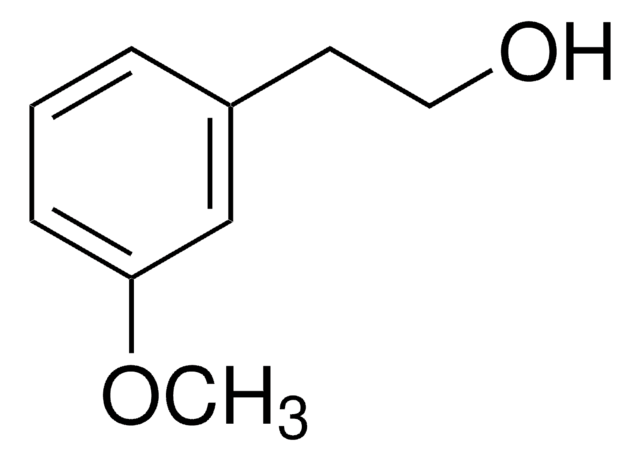
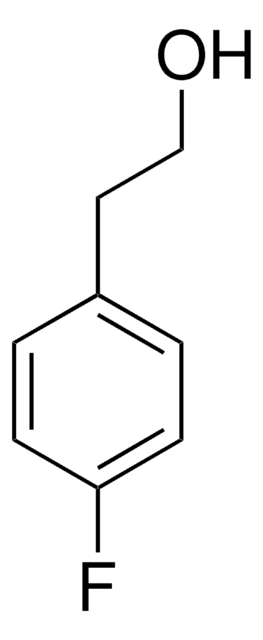
4-Bromophenethyl alcohol
99%
Synonym(s):
2-(4-Bromophenyl)ethanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4CH2CH2OH
CAS Number:
Molecular Weight:
201.06
Beilstein:
2079929
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.573 (lit.)
bp
138 °C/9 mmHg (lit.)
density
1.436 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
OCCc1ccc(Br)cc1
InChI
1S/C8H9BrO/c9-8-3-1-7(2-4-8)5-6-10/h1-4,10H,5-6H2
InChI key
PMOSJSPFNDUAFY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Bromophenethyl alcohol was used in the synthesis of 4-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenethoxy)quinazoline.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B Latli et al.
Chemical research in toxicology, 9(2), 445-450 (1996-03-01)
Two candidate photoaffinity probes are designed from 4-substituted quinazolines known to be potent insecticides/acaricides and NADH:ubiquinone oxidoreductase inhibitors acting at or near the rotenone site. 4-(11-Azidoundecyl-2-amino)quinazoline, based on the undecylamino analog SAN 548A as a prototype, was synthesized in 18%
Roman S Borisov et al.
Talanta, 200, 31-40 (2019-05-01)
This work highlights the discovered in-situ analytical reaction between primary/secondary alcohols and nitrogenous bases (pyridine, quinoline) that involves the substitution of hydroxyl groups for nitrogen-containing charged species and proceeds in an ionization region of Direct Analysis in Real Time mass
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(2R)-2-Phenyl-3,4-dihydro-2H-pyrimido[2,1-b][1,3]benzothiazole ≥95%](/deepweb/assets/sigmaaldrich/product/structures/302/833/59a06b8b-9e9d-4dc4-a4ed-a36ab5081792/640/59a06b8b-9e9d-4dc4-a4ed-a36ab5081792.png)