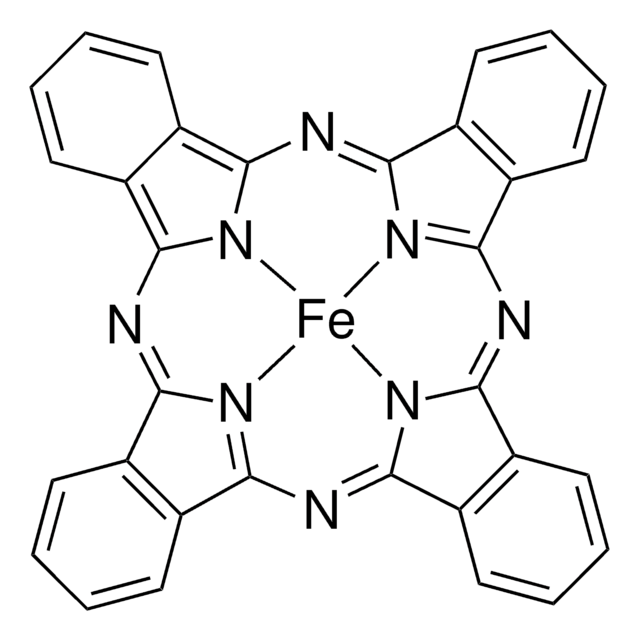
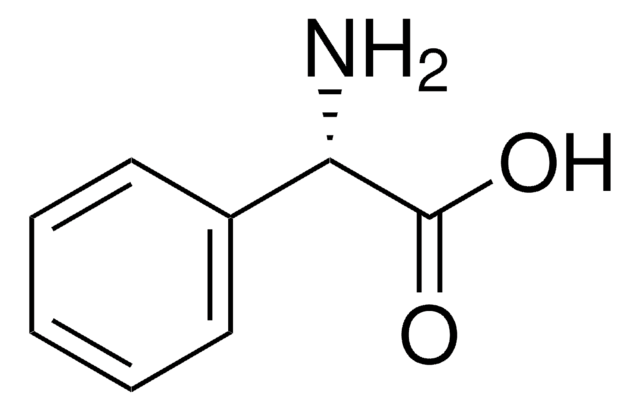
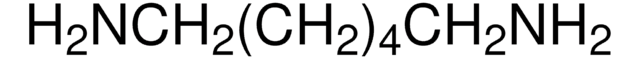15518
Boc-Inp-OH
≥99.0% (HPLC)
Synonym(s):
1-Boc-piperidine-4-carboxylic acid, Boc-isonipecotic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H19NO4
CAS Number:
Molecular Weight:
229.27
Beilstein:
5533442
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (HPLC)
form
solid
reaction suitability
reaction type: Boc solid-phase peptide synthesis
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)N1CCC(CC1)C(O)=O
InChI
1S/C11H19NO4/c1-11(2,3)16-10(15)12-6-4-8(5-7-12)9(13)14/h8H,4-7H2,1-3H3,(H,13,14)
InChI key
JWOHBPPVVDQMKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xunan Jing et al.
Nanoscale, 11(33), 15508-15518 (2019-08-09)
Although the collaborative therapy of chemotherapy (CT) and photodynamic therapy (PDT) is much more efficient for tumor treatment than monotherapies, premature leakage of drugs from nanocarriers and hypoxia in the tumor microenvironment (TME) result in systemic toxicity and suboptimal therapy
Jing Zhang et al.
Cell death and differentiation, 27(5), 1569-1587 (2019-10-28)
Microtubule-targeting agents (MTAs) are a class of most widely used chemotherapeutics and their mechanism of action has long been assumed to be mitotic arrest of rapidly dividing tumor cells. In contrast to such notion, here we show-in many cancer cell
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![tert-Butyl 4-[methoxy(methyl)carbamoyl]piperidine-1-carboxylate AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/262/563/6ebf90d4-42a2-4517-ba3e-8f112afe3661/640/6ebf90d4-42a2-4517-ba3e-8f112afe3661.png)