15461
Boc-Met-OSu
Synonym(s):
Boc-L-methionine hydroxysuccinimide ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
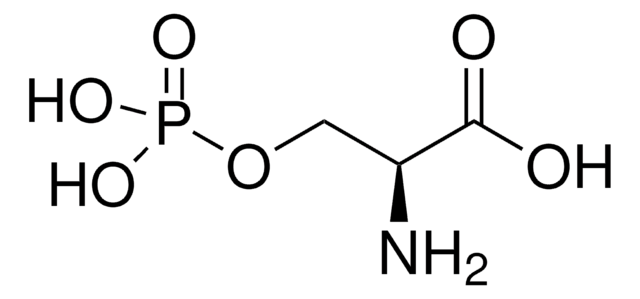
Empirical Formula (Hill Notation):
C14H22N2O6S
CAS Number:
Molecular Weight:
346.40
Beilstein:
4238574
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
optical activity
[α]20/D −21.5±2°, c = 2% in dioxane
reaction suitability
reaction type: Boc solid-phase peptide synthesis
ign. residue
≤0.05%
mp
120-128 °C
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
CSCC[C@H](NC(=O)OC(C)(C)C)C(=O)ON1C(=O)CCC1=O
InChI
1S/C14H22N2O6S/c1-14(2,3)21-13(20)15-9(7-8-23-4)12(19)22-16-10(17)5-6-11(16)18/h9H,5-8H2,1-4H3,(H,15,20)/t9-/m0/s1
InChI key
PCZJWSPKNYONIM-VIFPVBQESA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C W Basse et al.
The Journal of biological chemistry, 268(20), 14724-14731 (1993-07-15)
We have previously isolated glycopeptides derived from yeast invertase that acted as highly potent elicitors in suspension-cultured tomato cells, inducing ethylene biosynthesis and phenylalanine ammonia-lyase activity, and we have found that the high mannose oligosaccharides released from the pure glycopeptide
F Z Sheabar et al.
Chemical research in toxicology, 7(5), 650-658 (1994-09-01)
We report the chemical foundation for a new method to detect carcinogen-DNA adducts, which we have designated adduct detection by acylation with methionine (ADAM). The method is based on reaction of DNA adducts with a protected methionine derivative, (tert-butoxycarbonyl)-L-methionine N-hydroxysuccinimidyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service