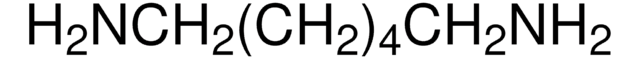15404
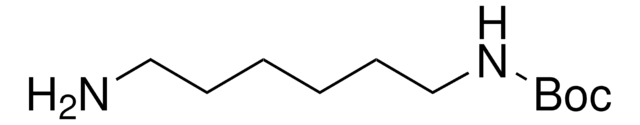
N-Boc-1,4-butanediamine
≥97.0% (GC/NT)
Synonym(s):
N-Boc-1,4-diaminobutane, tert-Butyl N-(4-aminobutyl)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
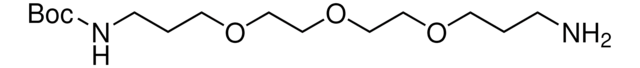
(CH3)3COCONH(CH2)4NH2
CAS Number:
Molecular Weight:
188.27
Beilstein:
1937878
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC/NT)
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.460
density
0.984 g/mL at 20 °C (lit.)
functional group
Boc
amine
SMILES string
NCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H20N2O2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7,10H2,1-3H3,(H,11,12)
InChI key
ZFQWJXFJJZUVPI-UHFFFAOYSA-N
Application
- Carboxy-Silane Coated Iron Oxide Nanoparticles: Details the application of N-Boc-1,4-butanediamine in modifying iron oxide nanoparticles for imaging and drug delivery (D Stanicki, S Boutry, S Laurent, et al., 2014). Access the article.
Other Notes
Preparation of pharmacologically active compounds. Preparation of spermidine analogues. Introduction of a C4-spacer.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y Shai et al.
Biochemistry, 28(11), 4801-4806 (1989-05-30)
In the present study we synthesize 18F-labeled insulin of high specific radioactivity. A new prosthetic group methodology, in which [18F]fluoride displaces a bromide group of 4-(bromomethyl)-benzoylamine intermediates, was used. The 4-(fluoromethyl)benzoyl product was chemically stable. 18F-Labeled insulin retains the essential
L I Kruse et al.
Journal of medicinal chemistry, 32(2), 409-417 (1989-02-01)
In an attempt to identify a soluble oncodazole analogue that could be easily formulated, a series of substituted oncodazoles was synthesized and evaluated for tubulin binding affinity, in vitro cytotoxicity against cultured mouse B-16 cells, and ability to prolong lifespan
Hongyan Guo et al.
Journal of medicinal chemistry, 45(10), 2056-2063 (2002-05-03)
Several iron chelators containing alpha,beta-unsaturated hydroxamic acid motifs appended to a citric acid platform were synthesized. Mycobacterium paratuberculosis was then challenged to grow in the presence of a panel of siderophores (mycobactin J, deferrioxamine B, acinetoferrin, and nannochelin A) and
M. McWatt, G.J. Boons et al.
European Journal of Organic Chemistry, 2535-2535 (2001)
H.H. Wassermann et al
Tetrahedron, 58, 7177-7177 (2002)
Articles
MRT - Mono-Boc-Protection of Diamines
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service