14353
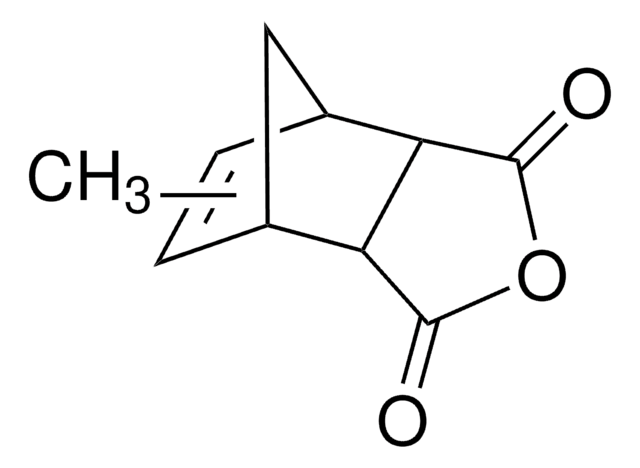
Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride
≥95.0%
Synonym(s):
5-Norbornene-2,3-dicarboxylic anhydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8O3
CAS Number:
Molecular Weight:
164.16
Beilstein:
150228
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
solubility
diethyl ether: 0.1 g/10 mL, clear, colorless
SMILES string
O=C1OC(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@@H]12
InChI
1S/C9H8O3/c10-8-6-4-1-2-5(3-4)7(6)9(11)12-8/h1-2,4-7H,3H2/t4-,5+,6-,7+
InChI key
KNDQHSIWLOJIGP-UMRXKNAASA-N
Looking for similar products? Visit Product Comparison Guide
General description
Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride reacts with methyl aminomethyllambertianate to give amide of bicyclo[2.2.1]heptan-1,2-dicarbocylic acid with a labdanoid substituent.
Application
- Sustainable chemical processes with Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride: Research by Kharitonov et al. (2012) explores synthetic transformations of higher terpenoids, employing Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride. Their work focuses on creating new cytotoxic agents, contributing to sustainable methodologies in organic synthesis (Kharitonov et al., 2012).
- Organic synthesis intermediate: Birchall et al. (2021) present a study on Himic Anhydride, utilizing a retro Diels-Alder reaction to demonstrate the application of Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride in teaching advanced organic laboratory techniques. This work includes an accompanying NMR study to further support the educational aspect of organic synthesis (Birchall et al., 2021).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Iu V Kharitonov et al.
Bioorganicheskaia khimiia, 38(1), 127-136 (2012-07-17)
Condensation of methyl 16-aminomethyllambertianate with N-Boc-omega-amino acids leads smoothly to 16-(N-Boc-aminononan)- and 16-(N-Boc-aminoundecan)amidomethyllabdanoids. The amide of bicyclo[2.2.1]heptan-1,2-dicarbocylic acid with a labdanoid substituent was obtained under the reaction of methyl aminomethyllambertianate with bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride. Intereaction of methyl 16-aminomethyllambertianate with chloroacetyl chloride
Richard Reece et al.
ACS applied materials & interfaces, 12(23), 25683-25692 (2020-05-15)
Considering the low specific capacitance of structural solid supercapacitors, which is due to the low ion diffusivity in solid electrolytes and the small specific surface area of some structural electrodes such as carbon fiber fabrics, novel structural supercapacitor designs are
An Yang et al.
Dental materials journal, 36(5), 560-565 (2017-06-02)
The aim of the study was to evaluate the effect of modifying polymethyl methacrylate (PMMA) denture base material with polyimide (PI) on its flexural property and biocompatibility. Low molecular weight (1,500 g/mol) PI was synthesized and small amount of PI
R Padhma Priya et al.
Journal of nanoscience and nanotechnology, 15(9), 6739-6746 (2015-12-31)
A new type of siloxane core modified phthalide cardo chain based polyimide (PI) was successfully prepared from siloxane core dianhydride and ether linked phenolphthalein diamine moiety. This PI was further modified with different weight percentages of multi-walled carbon nanotubes (MWCNTs)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service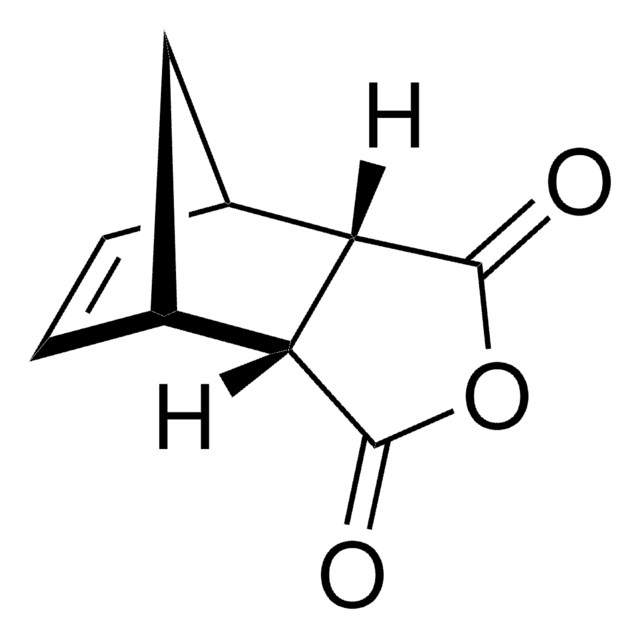




![Bicyclo[2.2.1]hept-5-ene-2-carboxaldehyde 95%](/deepweb/assets/sigmaaldrich/product/structures/420/624/80d9b7d0-cbbe-4841-b614-d06fdd69ba07/640/80d9b7d0-cbbe-4841-b614-d06fdd69ba07.png)