136964
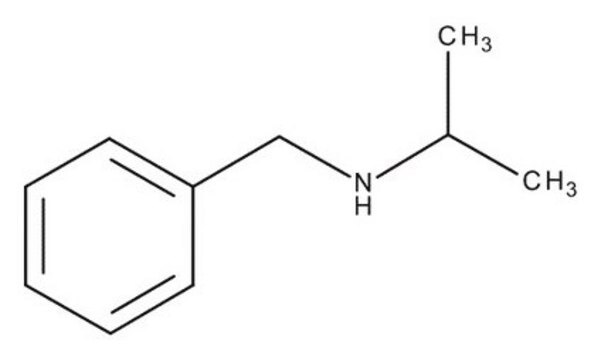
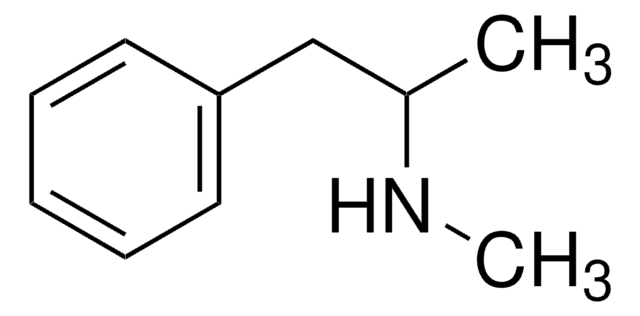
N-Isopropylbenzylamine
97%
Synonym(s):
N-Benzylisopropylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2NHCH(CH3)2
CAS Number:
Molecular Weight:
149.23
Beilstein:
2638437
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.502 (lit.)
bp
200 °C (lit.)
density
0.892 g/mL at 25 °C (lit.)
functional group
amine
phenyl
SMILES string
CC(C)NCc1ccccc1
InChI
1S/C10H15N/c1-9(2)11-8-10-6-4-3-5-7-10/h3-7,9,11H,8H2,1-2H3
InChI key
LYBKPDDZTNUNNM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
N-Isopropylbenzylamine forms amine adducts with magnesocene at ambient temperature in toluene.
Application
N-Isopropylbenzylamine was used as ligand in the preparation and characterization of bis(cyclopentadienyl)magnesium. It was also used in the synthesis of N-benzylideneisopropylamine-N-oxide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
161.6 °F - closed cup
Flash Point(C)
72 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
One-flask transformation of secondary amines to nitrones by oxidation with hydrogen peroxide mediated by triscetylpyridinium tetrakis oxodiperoxotungsto-phosphate (PCWP). Some mechanistic considerations.
Ballistreri FP, et al.
Tetrahedron, 48(40), 8677-8684 (1992)
Aibing Xia et al.
Journal of the American Chemical Society, 124(38), 11264-11265 (2002-09-19)
Magnesocene adducts of alkylamines were prepared and characterized. Treatment of 3-amino-2,4-dimethylpentane, isopropylamine, tert-butylamine, benzylamine, or N-isopropylbenzylamine with magnesocene at ambient temperature in toluene afforded the amine adducts Cp2Mg(NH2CH(CH(CH3)2)2) (91%), Cp2Mg(NH2iPr) (80%), Cp2Mg(NH2tBu) (67%), Cp2Mg(NH2CH2Ph) (80%), and Cp2Mg(NH(CH(CH3)2)(CH2C6H5)) (91%). These adducts
O Brüggemann
Biomolecular engineering, 18(1), 1-7 (2001-06-29)
Molecular imprinting is a way of creating polymers bearing artificial receptors. It allows the fabrication of highly selective plastics by polymerizing monomers in the presence of a template. This technique primarily had been developed for the generation of biomimetic materials
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 136964-25G | 4061836825201 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service