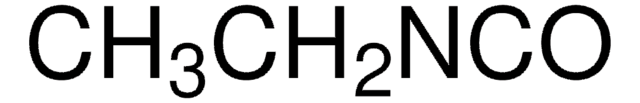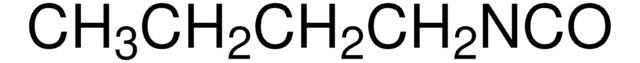133280
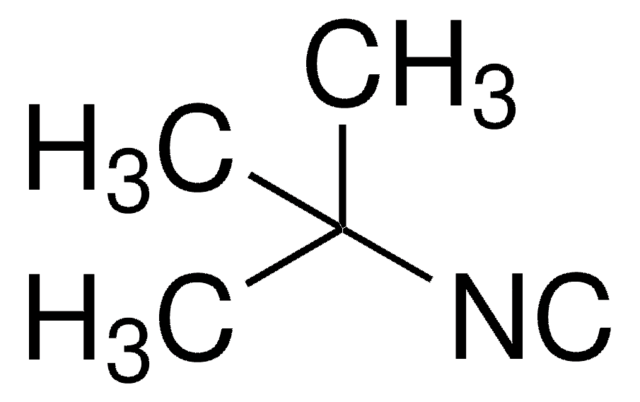
Butyl isocyanide
97%
Synonym(s):
1-Butylisonitrile, 1-Isocyanobutane, Butyl isonitrile, n-Butyl isocyanide, n-Butyl isonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3NC
CAS Number:
Molecular Weight:
83.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.396 (lit.)
density
0.795 g/mL at 25 °C (lit.)
functional group
amine
isonitrile
storage temp.
2-8°C
SMILES string
CCCC[N+]#[C-]
InChI
1S/C5H9N/c1-3-4-5-6-2/h3-5H2,1H3
InChI key
FSBLVBBRXSCOKU-UHFFFAOYSA-N
Related Categories
Application
Butyl isocyanide was used to study the crystal structure of carbon monoxide dehydrogenases-II isolated from Carboxydothermus hydrogenoformans. It was used to probe the mechanism of NO activation of the hemoprotein soluble guanylate cyclase. .
Biochem/physiol Actions
Butyl isocyanide forms complex with ferric and ferrous iron of the heme in cytochrome P450.
Other Notes
May form haziness and/or precipitate with no loss in purity.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
69.8 °F - closed cup
Flash Point(C)
21 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T H Tahirov et al.
Nature structural biology, 3(5), 459-464 (1996-05-01)
We have determined the structure of n-butylisocyanide-bound Rhodobacter capsulatus cytochrome c'. This is the first example of a ligand-bound structure of a class IIa cytochrome c. Compared with the structure of native cytochrome c', there are significant conformational changes of
D S Lee et al.
Biochemistry, 40(9), 2669-2677 (2001-03-22)
Alkyl-isocyanides are able to bind to both ferric and ferrous iron of the heme in cytochrome P450, and the resulting complexes exhibit characteristic optical absorption spectra. While the ferric complex gives a single Soret band at 430 nm, the ferrous
D Barrick et al.
Biochemistry, 40(13), 3780-3795 (2001-04-13)
The linkage between the proximal histidines and the proximal polypeptide in normal adult human hemoglobin (Hb A) has been proposed to play a major role in transmitting allosteric effects between oxygen binding sites [Perutz, M. F. (1970) Nature 228, 726-734].
C Mouro et al.
European journal of biochemistry, 267(1), 216-221 (1999-12-22)
An 1H-NMR study of ferric cytochrome P450cam in different paramagnetic states was performed. Assignment of three heme methyl resonances of the isocyanide adduct of cytochrome P450 in the ferric low-spin state was recently performed using electron exchange in the presence
Y Imai et al.
Biochimica et biophysica acta, 1337(1), 66-74 (1997-01-04)
Heme-external ligand interactions of P-450nor were examined spectrophotometrically and compared with those of other P-450s. Most nitrogenous ligands induced type II spectral changes on binding to ferric P-450nor, as did other P-450s. In contrast with other P-450s, 2-methylpyridine and 1-butanol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service