117757
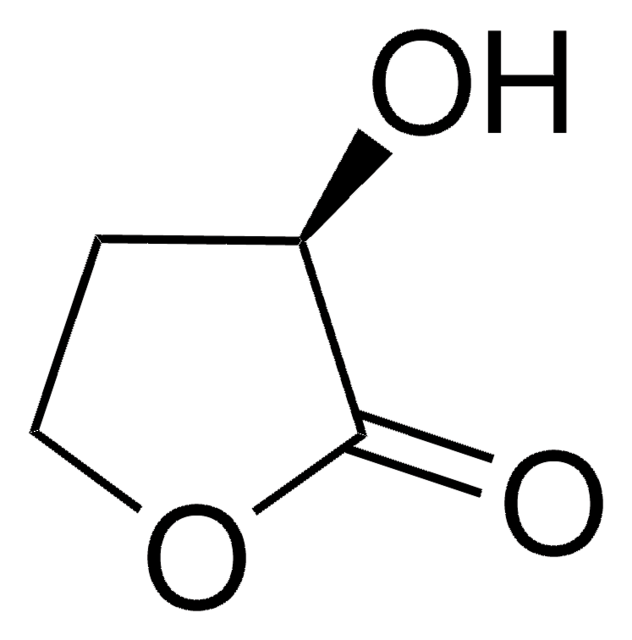
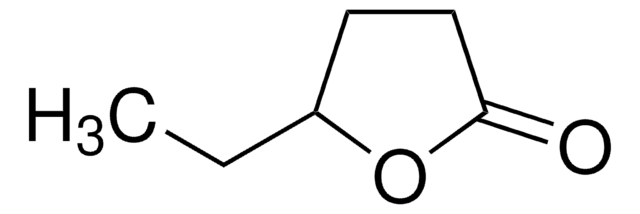
α-Methyl-γ-butyrolactone
98%
Synonym(s):
4,5-Dihydro-3-methyl-2(3H)-furanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8O2
CAS Number:
Molecular Weight:
100.12
Beilstein:
80418
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.432 (lit.)
bp
78-81 °C/10 mmHg (lit.)
solubility
THF: soluble
functional group
ester
SMILES string
CC1CCOC1=O
InChI
1S/C5H8O2/c1-4-2-3-7-5(4)6/h4H,2-3H2,1H3
InChI key
QGLBZNZGBLRJGS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
α-Methyl-γ-butyrolactone undergoes benzylation to give racemic α-benzyl-α-methyl-γ-butyrolactone.
Application
α-Methyl-γ-butyrolactone was used as model compound in Bracketing experiments to investigate the thermodynamically favored site of reaction of pilocarpine.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
163.4 °F - closed cup
Flash Point(C)
73 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Satterfield et al.
Journal of the American Society for Mass Spectrometry, 10(3), 209-216 (1999-03-09)
Analysis of the sites of reaction of a biologically important compound, pilocarpine, a molecule with imidazole and butyrolactone rings connected by a methylene bridge, has been accomplished in a quadrupole ion trap with the aim of characterizing its structure/reactivity relationships.
Eric B Gonzales et al.
The Journal of pharmacology and experimental therapeutics, 309(2), 677-683 (2004-01-27)
Alkyl-substituted butyrolactones have both inhibitory and stimulatory effects on GABA(A) receptors. Lactones with small alkyl substitutions at the alpha-position positively modulate the channel, whereas beta-substituted lactones tend to inhibit the GABA(A) receptor. These compounds mediate inhibition through the picrotoxin site
Hagai Tavori et al.
Bioorganic & medicinal chemistry, 16(15), 7504-7509 (2008-06-24)
Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters and lactones, whereas the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service