54096-U
Supelclean™ ENVI-Carb/NH2 SPE Tube
bed A 500 mg (Supelclean ENVI-Carb), bed B 500 mg (Supelclean LC-NH2), volume 20 mL, pk of 200
About This Item
Recommended Products
material
polypropylene tube
product line
Supelclean™
composition
bed A, 500 mg (Supelclean ENVI-Carb)
bed B, 500 mg (Supelclean LC-NH2)
packaging
pk of 200
technique(s)
solid phase extraction (SPE): suitable
volume
20 mL
matrix active group
aminopropyl phase
carbon phase
application(s)
food and beverages
separation technique
ion exchange
reversed phase
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sample Matrix Compatibility: Organic or aqueous solutions
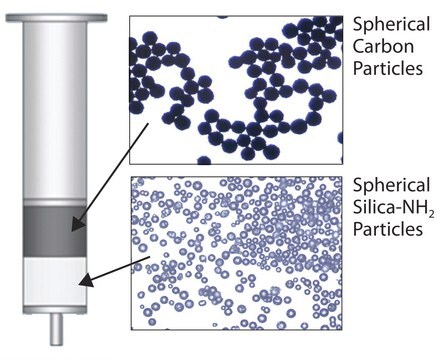
- Dual layer SPE tube that contains both Supelclean ENVI-Carb (upper layer) & LC-NH2 (lower layer) SPE sorbents (separated by PE frit)
- Developed to offer superior clean up when conducting multi-residue pesticide analysis from food (e.g. agricultural products, meats, etc.).
- ENVI-carb has a strong affinity towards planar molecules, and can isolate/remove pigments (e.g., chlorophyll and carotinoids) and sterols commonly present in foods and natural products
- Supelclean LC-NH2 is an aminopropyl phase that retains fatty acids, organic acids, and some polar pigments and sugars common in food matrices
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
SPE retention mechanism in this case is based on the electrostatic attraction of charged functional groups of the analyte(s) to oppositely charged functional groups on the sorbent.
Reversed-phase interaction will retain most molecules with hydrophobic character; it is very useful for extracting analytes that are very diverse in structure within the same sample.
Protocols
Retention occurs through polar interaction between the sorbent and analytes. Typical sample matrices that can be employed in normal-phase SPE include hydrocarbon or fatty oils diluted in a solvent like hexane, isooctane, chlorinated solvent, THF, diethyl ether, or ethyl acetate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service