S8559
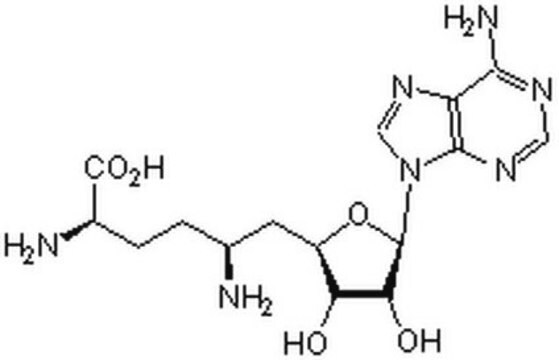
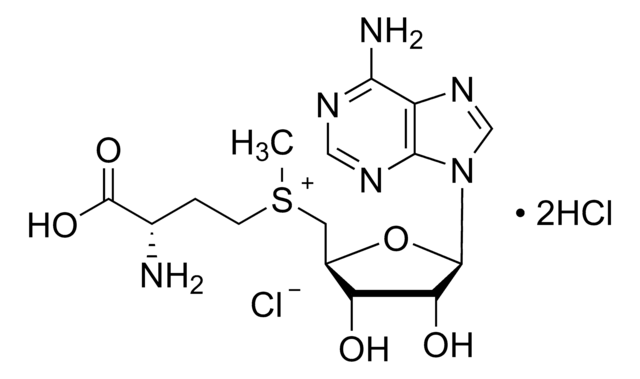
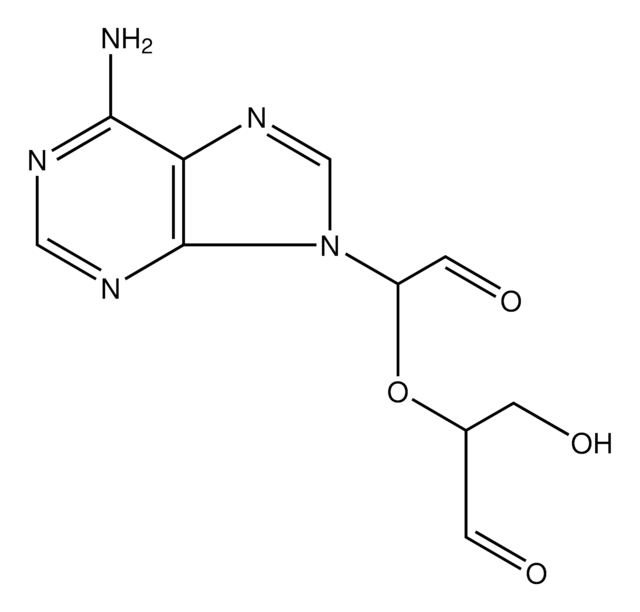
Sinefungin
95% (HPLC), powder, methylation of bases in DNA & RNA inhibitor
Synonym(s):
5′-Deoxy-5′-(1,4-diamino-4-carboxybutyl)adenosine, A-9145, Adenosylornithine, Antibiotic 32232RP
About This Item
Recommended Products
Product Name
Sinefungin, 95% (HPLC), powder
Quality Level
Assay
95% (HPLC)
form
powder
color
white to yellow
solubility
H2O: complete 20 mg/ml, clear, colorless to light yellow
H2O: soluble
antibiotic activity spectrum
neoplastics
Mode of action
DNA synthesis | interferes
enzyme | inhibits
storage temp.
2-8°C
SMILES string
N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1
InChI key
LMXOHSDXUQEUSF-YECHIGJVSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Methylation inhibition by sinefugin is often accompanied by an altered rate of cytosine deamination that is coupled to transition mutation in the DNA. Sinefugin inhibits Epstein-Barr viral activity and this inhibition is related to the change in DNA methylation and gene expression. It can cause a rate change in several restriction DNA endonuclease activities, including Mme I, which is not connected to the inhibition of the methytransferase activity.
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service