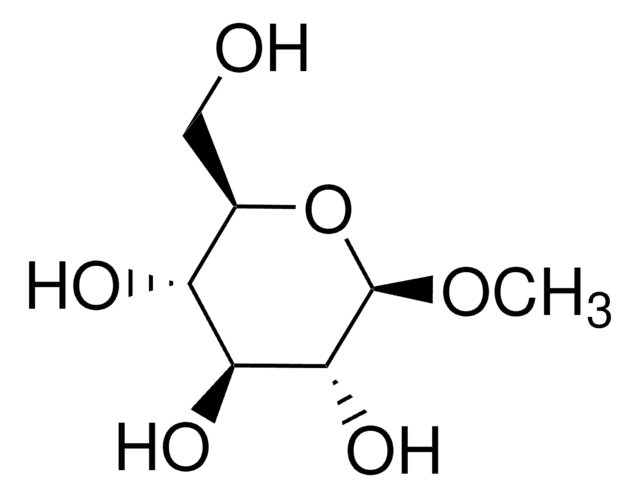
P6501
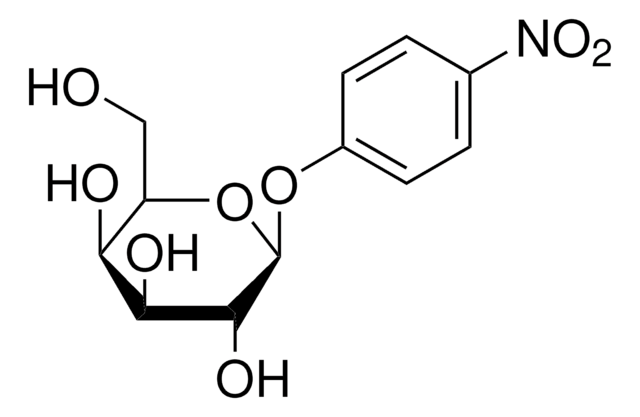
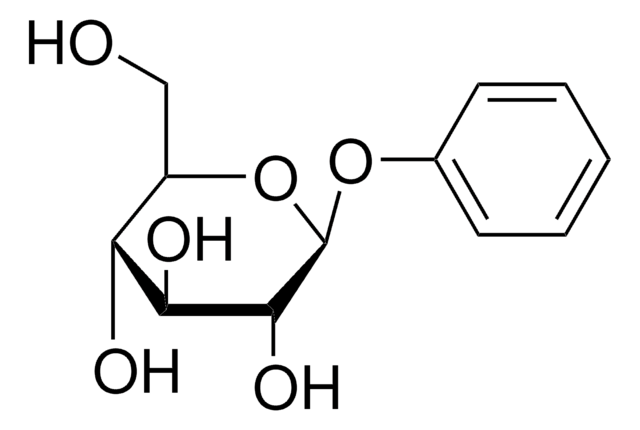
Phenyl-β-D-galactopyranoside
≥98% (TLC)
Synonym(s):
P-Gal, Phenyl β-D-galactoside
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H16O6
CAS Number:
Molecular Weight:
256.25
Beilstein:
87518
EC Number:
MDL number:
UNSPSC Code:
41141627
PubChem Substance ID:
NACRES:
NA.52
Recommended Products
grade
for mineral oil production sites, untaxed
Quality Level
Assay
≥98% (TLC)
form
powder
storage temp.
−20°C
SMILES string
OC[C@H]1O[C@@H](Oc2ccccc2)[C@H](O)[C@@H](O)[C@H]1O
InChI
1S/C12H16O6/c13-6-8-9(14)10(15)11(16)12(18-8)17-7-4-2-1-3-5-7/h1-5,8-16H,6H2/t8-,9+,10+,11-,12-/m1/s1
InChI key
NEZJDVYDSZTRFS-YBXAARCKSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Phenyl-β-D-galactopyranoside is used as a substrate for detecting β-galactosidase enzymatic activity.
Application
Phenyl-β-D-galactopyranoside has been used in mutant phage screening.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ziyu Deng et al.
International journal of biological macromolecules, 137, 69-76 (2019-07-02)
β-Galactosidase (β-Gal) as dietary supplement has the ability to alleviate symptoms of lactose intolerance. This study investigated the ability of oligosaccharides to protect β-Gal against heat stress. Four kinds of oligosaccharides including Isomalto-oligosaccharides (IMO), Xylo-oligosaccharides (XOS), Konjac-oligosaccharides (KOS), and Mycose
Daniel D Mitchell et al.
Bioorganic & medicinal chemistry, 12(5), 907-920 (2004-02-26)
With the aim of developing high-affinity mono and multivalent antagonists of cholera toxin (CT) and Escherichia coli heat-labile enterotoxin (LT) we are using the galactose portion of the natural receptor ganglioside GM1 as an anchoring fragment in structure-based inhibitor design
J Jiao et al.
Mutation research, 372(1), 141-145 (1996-11-11)
lacZ gene mutations in the transgenic Muta Mmouse can be detected by two different selection systems. While mutant frequencies recovered by phenyl-beta-D-galactoside (P-gal) selection are comparable with those obtained using 5-bromo-4-chloro-3-indolyl beta-D-galactopyranoside (X-gal) as substrate for beta-galactosidase, there may still
A strong genotoxic effect in mouse skin of a single painting of coal tar in hairless mice and in Muta? Mouse
Thein N, et al.
Mutation Research. Genetic Toxicology and Environmental Mutagenesis, 468(2), 117-124 (2000)
M E Dollé et al.
Mutagenesis, 14(3), 287-293 (1999-06-22)
The plasmid-based transgenic mouse model, which uses the lacZ gene as the target for mutation, is sensitive to a wide range of in vivo mutations, ranging from point mutations to insertions and deletions extending far into the mouse genome. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service