P3988
20S Proteasome Fraction from rabbit
≥95% (SDS-PAGE), solution
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
biological source
rabbit
Quality Level
Assay
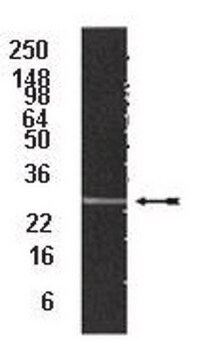
≥95% (SDS-PAGE)
form
solution
mol wt
700 kDa
UniProt accession no.
application(s)
cell analysis
shipped in
dry ice
storage temp.
−70°C
Gene Information
rabbit ... PSMA1(100352563) , PSMB1(100343622)
General description
Manufactured for Sigma by Boston Biochem., Inc.
Application
20S proteasome fraction from rabbit has been used as a positive control to determine proteasome activity.
Biochem/physiol Actions
Catalytic core of the 26S proteasome that degrades polyubiquitinated proteins.
Hydrolyzes various peptide substrates and proteins with broad specificity in a non-ATP dependent process.
Physical form
Solution in 50 mM HEPES, pH 7.6, 150 mM sodium chloride, and 1 mM DTT, pH 7.6.
Storage Class Code
10 - Combustible liquids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R L Stein et al.
Biochemistry, 35(13), 3899-3908 (1996-04-02)
In this paper, we report kinetic studies for the chymotryptic activity of the 20S proteasome. Major observations include the following: (1) Reaction progress curves that are recorded at concentrations of Suc-Leu-Leu-Val-Tyr-AMC greater than about 40 microM are biphasic and characterized
Examination of `lipotoxicity? in skeletal muscle of high-fat fed and ob/ob mice
S.M. Turpin
The Journal of Physiology (2009)
R Hough et al.
The Journal of biological chemistry, 262(17), 8303-8313 (1987-06-15)
We have purified two high molecular weight proteases approximately 400-fold from rabbit reticulocyte lysate. Both enzymes hydrolyze 125I-alpha-casein and 4-methylcoumaryl-7-amide peptides with tyrosine, phenylalanine, or arginine at the P1 position. Both are inhibited by hemin, thiol reagents, chymostatin, and leupeptin.
S M Turpin et al.
The Journal of physiology, 587(Pt 7), 1593-1605 (2009-02-11)
Excess lipid accumulation resulting from an elevated supply of plasma fatty acids is linked to the pathogenesis of the metabolic syndrome and heart disease. The term 'lipotoxicity' was coined to describe how lipid accumulation leads to cellular dysfunction and death
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service