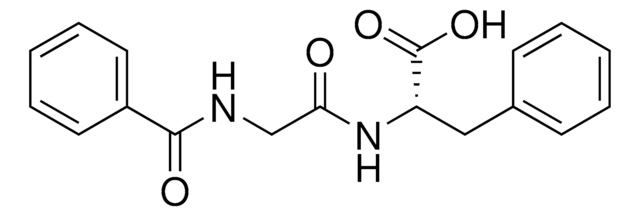
L9125
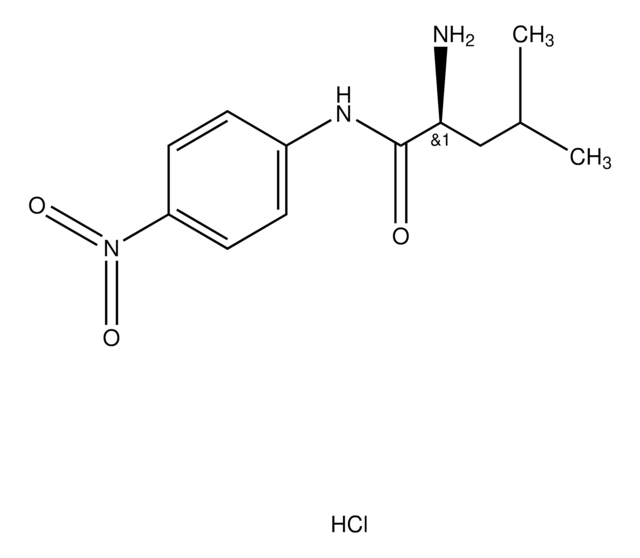
L-Leucine-p-nitroanilide
leucine aminopeptidase substrate, chromogenic, ≥98% (TLC), powder
Synonym(s):
L-Leucine-4-nitroanilide
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C12H17N3O3
CAS Number:
Molecular Weight:
251.28
Beilstein:
2218433
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Product Name
L-Leucine-p-nitroanilide, leucine aminopeptidase substrate
Quality Level
Assay
≥98% (TLC)
form
powder
solubility
ethanol: 50 mg/mL
storage temp.
2-8°C
SMILES string
CC(C)C[C@H](N)C(=O)Nc1ccc(cc1)[N+]([O-])=O
InChI
1S/C12H17N3O3/c1-8(2)7-11(13)12(16)14-9-3-5-10(6-4-9)15(17)18/h3-6,8,11H,7,13H2,1-2H3,(H,14,16)/t11-/m0/s1
InChI key
AXZJHDNQDSVIDR-NSHDSACASA-N
Looking for similar products? Visit Product Comparison Guide
Application
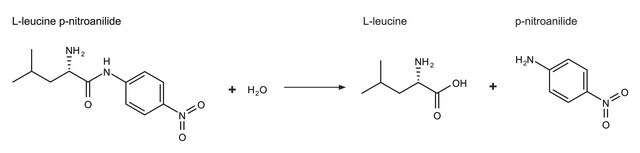
L-Leucine-p-nitroanilide has been used as substrate
- for the determination of intestinal leucine aminopeptidase activity of juvenile rainbow trout (Oncorhynchus mykiss)
- in insulin-regulated aminopeptidase inhibition assay
- for determining exopeptidase activity
Substrates
Substrate for the colorimetric determination of leucine aminopeptidase.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Identification of drug-like inhibitors of insulin-regulated aminopeptidase through small-molecule screening
Engen K, et al.
Assay and Drug Development Technologies, 14(3), 180-193 (2016)
W Bawab et al.
The Biochemical journal, 286 ( Pt 3), 967-975 (1992-09-15)
Aminopeptidase activities were identified in extracts of kidney, ovotestis, head ganglia, heart and haemolymph of Aplysia californica. These enzyme preparations hydrolysed [3H][Leu]enkephalin at the Try-1-Gly-2 bond as determined by h.p.l.c. analysis of cleavage products. In all these tissues, enkephalin-degrading aminopeptidase
K W Garren et al.
Pharmaceutical research, 6(11), 966-970 (1989-11-01)
The simultaneous diffusion and metabolism of the D- and L-isomers of the aminopeptidase substrate, leucine-p-nitroanilide (LPNA), were examined in vitro in the hamster cheek pouch. L-LPNA was completely hydrolyzed during diffusion across the cheek pouch, whereas D-LPNA crossed the cheek
Effects of fish meal replacement with meat and bone meal using garlic (Allium sativum) powder on growth, feeding, digestive enzymes and apparent digestibility of nutrients and fatty acids in juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792)
Esmaeili M, et al.
Aquaculture Nutrition, 23(6), 1225-1234 (2017)
I M Cesari et al.
The Journal of parasitology, 69(2), 280-284 (1983-04-01)
An aminopeptidase activity capable of hydrolysing leucine 4-nitroanilide and alanine 4-nitroanilide at pH 7.0 was detected in saponin-CaCl2 extracts and homogenates of adult Schistosoma mansoni. The extracts were also capable of acting on synthetic dipeptides at the same pH, preferentially
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service