E7500
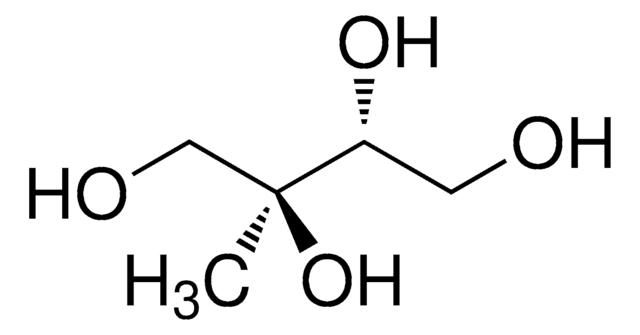
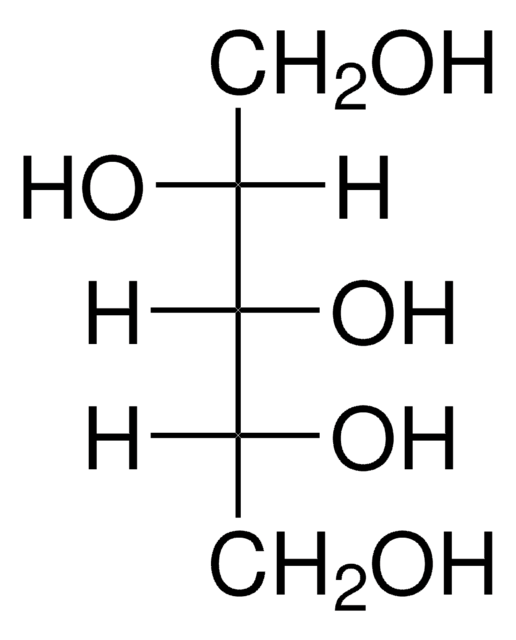
meso-Erythritol
≥99% (GC)
Synonym(s):
1,2,3,4-Butanetetrol, meso-1,2,3,4-Tetrahydroxybutane, i-Erythritol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
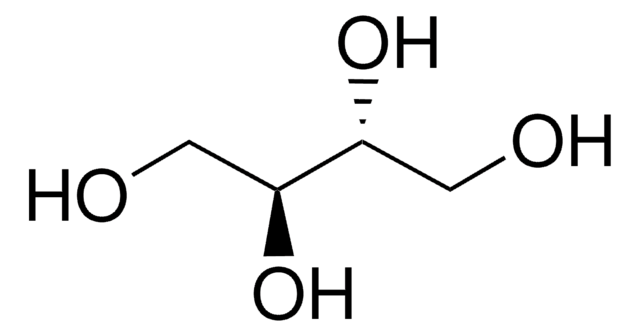
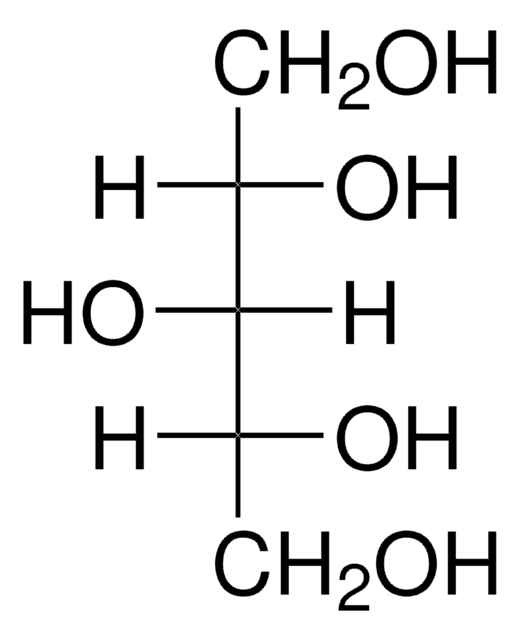
HOCH2[CH(OH)]2CH2OH
CAS Number:
Molecular Weight:
122.12
Beilstein:
1719753
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥99% (GC)
form
powder
sweetness
0.7 × sucrose
color
white
bp
329-331 °C (lit.)
mp
118-120 °C (lit.)
solubility
H2O: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
OC[C@@H](O)[C@@H](O)CO
InChI
1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2/t3-,4+
InChI key
UNXHWFMMPAWVPI-ZXZARUISSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Meso-Erythritol (i-Erythritol) is a precursor to the important tanning agent L-erythrulose which can be commercially generated by Gluconobacter oxydans DSM 7145. Meso-Erythritol may be used to support the carbon source growth requirement of Gluconobacter bacteria. It is an aliphatic poly-alcohol used in the chemical analysis of the reactivity of various radicals and radical anions. Meso-Erythritol may be used in encapsulated phase change materials (PCMs) to study its potential use in microelectronics cooling applications. It may be used as a reference compound in purification and analytical procedures developed to test fermentation broths.
Biochem/physiol Actions
Allelic variation of the Tas1r3 gene affects behavioral taste responses to this sugar alcohol, suggesting that it is a T1R3 receptor ligand.
Other Notes
To gain a comprehensive understanding of our extensive range of Sugar alcohols for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Man-Yeon Choi et al.
Journal of economic entomology, 112(2), 981-985 (2018-11-30)
Previous studies have demonstrated various combinations of non-nutritive erythritol and sucrose having detrimental effects on Drosophila suzukii (Matsumura). Fly mortality is likely caused by 1) starvation from feeding on non-metabolizable erythritol; and 2) physiological imbalance with abnormally high osmotic pressure
H Hino et al.
Acta dermato-venereologica, 62(3), 185-191 (1982-01-01)
Keratinocytes were dissociated from normal human adult epidermis with clostridial collagenase, dithio-erythritol and trypsin, and cultured. Immediately after this, no connection was seen between contiguous cells. Ruptured desmosomes, with masses of tonofilaments and distinct attachment plaques were still left on
Freek Spitaels et al.
International journal of systematic and evolutionary microbiology, 64(Pt 4), 1134-1141 (2013-12-26)
Three strains, LMG 27748(T), LMG 27749 and LMG 27882 with identical MALDI-TOF mass spectra were isolated from samples taken from the brewery environment. Analysis of the 16S rRNA gene sequence of strain LMG 27748(T) revealed that the taxon it represents
Karnjapan Janthawornpong et al.
Journal of the American Chemical Society, 135(5), 1816-1822 (2013-01-16)
The MEP pathway, which is absent in animals but present in most pathogenic bacteria, in the parasite responsible for malaria and in plant plastids, is a target for the development of antimicrobial drugs. IspH, an oxygen-sensitive [4Fe-4S] enzyme, catalyzes the
Emmanuel Jean Teinkela Mbosso et al.
Chemistry & biodiversity, 10(2), 224-232 (2013-02-19)
A chemical investigation of the Glyphaea brevis leaves and of the Monodora myristica fruits led to the identification of thirteen compounds, seven linear long-chain aliphatic compounds, 1, 2, 4, 6, and 9-11, three steroids, 3a, 3b, and 7, two triterpenes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service