D4943
Dipeptidyl Peptidase IV human
recombinant, expressed in baculovirus infected Sf9 cells, pkg of ≥1.0 units/vial, ≥10 units/mg protein
Synonym(s):
CD26, DPPIV, Dipeptidyl aminopeptidase IV, Glycoprotein GP110
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in baculovirus infected Sf9 cells
Quality Level
form
solution
specific activity
≥10 units/mg protein
mol wt
105 kDa
packaging
pkg of ≥1.0 units/vial
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... DPP4(1803)
Looking for similar products? Visit Product Comparison Guide
General description
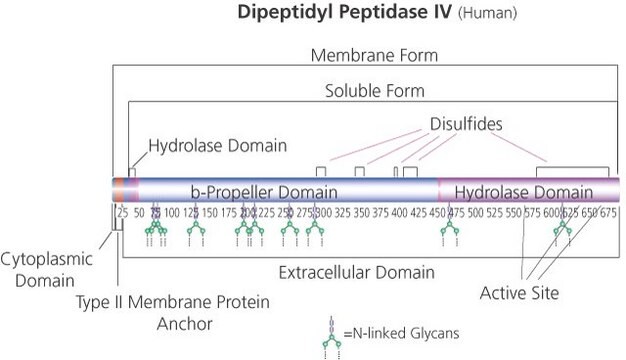
C-terminal histidine-tagged. Soluble form (residues 29-766) MW 105 kDa
Application
Human dipeptidyl peptidase IV has been used to study interactive hemodynamic effects of its inhibition and angiotensin-converting enzyme inhibition in humans. Human dipeptidyl peptidase IV has also been used in a study that informed the understanding of Hymenoptera venom allergies.
The enzyme from Sigma has been used to study the LC-MS (liquid chromatography-mass spectrometry) based assay method for DPP-IV inhibitor screening and substrate discovery.
Biochem/physiol Actions
DPPIV has a post-proline dipeptidyl aminopeptidase activity that hydrolyzes N-terminal dipeptides from the unsubstituted N-terminus of peptides with the sequence of X-Pro-Z and X-Ala-Z. The optimum pH is found to be 7.4-8.7. DPPIV is involved in the regulation of several important physiological processes such as immune functions, inflammation, CNS, endocrine functions, bone marrow mobilization, cancer growth, cell adhesion, glucose hemostasis and sepsis/severe infection.
Native DPPIV is a ubiquitous type II transmembrane glycoprotein and a serine protease of the S9 prolyl-oligopeptidase family. In vivo, it is synthesized with a signal peptide, which functions as the membrane anchoring domain. There is an 88% sequence homology between the human and porcine kidney enzymes. Both exist as homodimers with a subunit molecular weight of ~30 kDa. The high mannose 100 kDa DPPIV precursor is processed in the Golgi to yield a 124 kDa heavily N-and O-linked mature glycoprotein. It is then sorted to the apical membrane through the concerted action of both N- and O-linked glycans and its association with lipid microdomains. The porcine enzyme contains 18.3% carbohydrates, which the glycan composition is 0.9% fucose, 3.4% mannose, 5.1% galactose, 8.2% glucosamine, and 0.7% sialic acid. DPPIV is highly expressed on endothelial cells, epithelial cells, and lymphocytes. It is also present in plasma in its soluble form.
Unit Definition
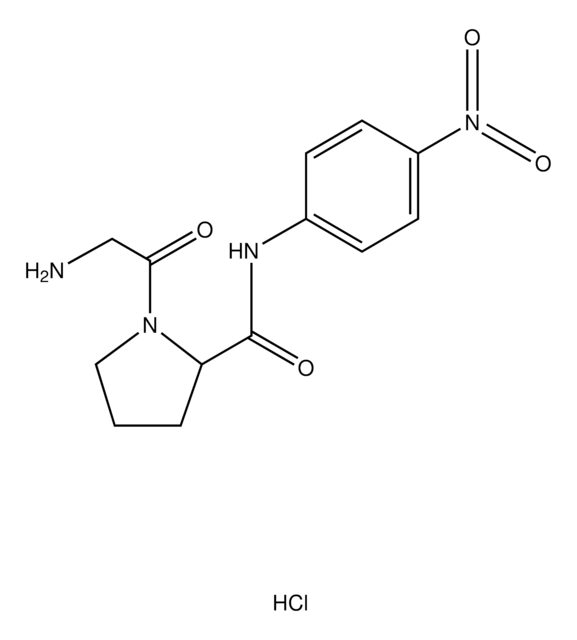
One unit will produce 1.0 μmole of p-nitroaniline from Gly-L-Pro p-nitroanilide per min in 100 mM Tris-HCl at pH 7.6 at 37 °C.
Physical form
Supplied as a solution in 10 mM Tris-HCl, pH 7.6, 200 mM NaCl, 1 mM EDTA and 10% glycerol.
Other Notes
View more information on Dipeptidyl Peptidase IV at www.sigma-aldrich.com/enzymeexplorer.
inhibitor
substrate
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
LC-MS based assay method for DPP-IV inhibitor screening and substrate discovery.
Liu, J., Cheng, X., & Fu, L.
Analytical Methods : Advancing Methods and Applications, 4(6), 1797-1805 (2012)
Ryugen Takahashi et al.
Frontiers in oncology, 11, 714527-714527 (2021-09-08)
Radical resection is the only curative treatment for pancreatic cancer, which is a life-threatening disease. However, it is often not easy to accurately identify the extent of the tumor before and during surgery. Here we describe the development of a
Mark D Gorrell
Clinical science (London, England : 1979), 108(4), 277-292 (2004-12-09)
DP (dipeptidyl peptidase) IV is the archetypal member of its six-member gene family. Four members of this family, DPIV, FAP (fibroblast activation protein), DP8 and DP9, have a rare substrate specificity, hydrolysis of a prolyl bond two residues from the
Petr Busek et al.
The international journal of biochemistry & cell biology, 36(3), 408-421 (2003-12-23)
Post-translational modification of proteins is an important regulatory event. Numerous biologically active peptides that play an essential role in cancerogenesis contain an evolutionary conserved proline residue as a proteolytic-processing regulatory element. Proline-specific proteases could therefore be viewed as important "check-points".
Ivan O Maslov et al.
Pharmaceuticals (Basel, Switzerland), 15(3) (2022-03-27)
Compounds that contain (R)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid substituted with bicyclic amino moiety (2-aza-bicyclo[2.2.1]heptane) were designed using molecular modelling methods, synthesised, and found to be potent DPP-4 (dipeptidyl peptidase-4) inhibitors. Compound 12a (IC50 = 16.8 ± 2.2 nM), named neogliptin, is a more
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service