B1403
Boc-L-alaninal
≥98%
Synonym(s):
Boc-L-alanine aldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H15NO3
CAS Number:
Molecular Weight:
173.21
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
Boc-L-alaninal, ≥98%
Quality Level
Assay
≥98%
form
powder
color
white
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
C[C@H](NC(=O)OC(C)(C)C)C=O
InChI
1S/C8H15NO3/c1-6(5-10)9-7(11)12-8(2,3)4/h5-6H,1-4H3,(H,9,11)/t6-/m0/s1
InChI key
OEQRZPWMXXJEKU-LURJTMIESA-N
Gene Information
human ... CTSK(1513)
Related Categories
Application
Boc-L-alaninal is used as a reagent for organic synthesis of C(26)-C(32) Oxazole Fragment of Calyculin C and other molecules.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Petri M. Pihko et al.
The Journal of organic chemistry, 63(1), 92-98 (2001-10-25)
The synthesis of the C(26)-C(32) oxazole fragment 4 and its C(32) epimer 20 of serine/threonine protein phosphatase PP1 and PP2A inhibitor calyculin C is presented. The syn methyl arrangement in 4 was established through cyclic stereocontrol. Several methods for oxidizing
Johann Chan et al.
The Journal of organic chemistry, 76(6), 1767-1774 (2011-02-09)
Two new, reliable syntheses of a pyrido[2,3-d]-pyrimidine inhibitor of the CXCR3 receptor are described. A nine-step synthesis of the CXCR3 inhibitor (1) from 2-aminonicotinic acid was demonstrated on a multikilogram scale and incorporates a classic resolution to deliver the enantioenriched
James A Marshall et al.
Organic letters, 7(8), 1593-1596 (2005-04-09)
[reaction: see text] Additions of chiral allenylzinc and indium reagents to N-Boc alaninal were examined as a possible route to a C20-C26 segment of superstolide A. Allenylzinc reagents, prepared in situ by palladiozincation of (R)- and (S)-5-pivalyloxy-3-butyn-2-ol mesylate, showed excellent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service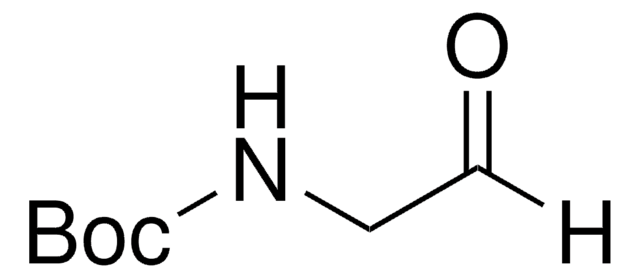
![3-[(Benzyloxycarbonyl)amino]propionaldehyde 95%](/deepweb/assets/sigmaaldrich/product/structures/408/203/100fb0f0-7072-41be-b6e0-2857cdc324ee/640/100fb0f0-7072-41be-b6e0-2857cdc324ee.png)