A7219
L-Aspartic acid
from non-animal source, meets EP, USP testing specifications, suitable for cell culture, 98.5-101.0%
Synonym(s):
(S)-(+)-Aminosuccinic acid, (S)-Aminobutanedioic acid
About This Item
Recommended Products
biological source
non-animal source
Quality Level
Agency
meets EP testing specifications
meets USP testing specifications
Assay
98.5-101.0%
form
powder
technique(s)
cell culture | mammalian: suitable
impurities
endotoxin, tested
color
white to off-white
mp
>300 °C (dec.) (lit.)
solubility
1 M HCl: 100 mg/mL
SMILES string
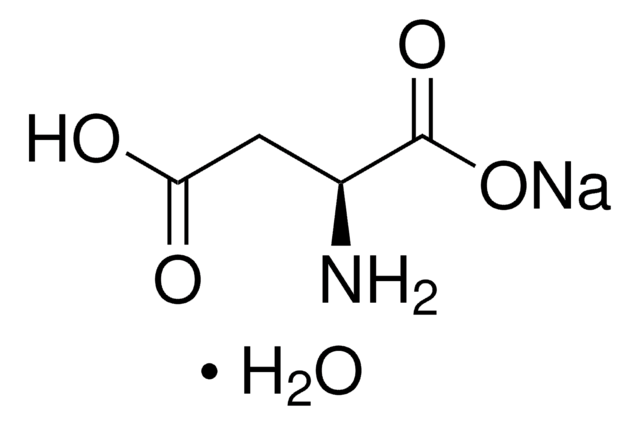
N[C@@H](CC(O)=O)C(O)=O
InChI
1S/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)/t2-/m0/s1
InChI key
CKLJMWTZIZZHCS-REOHCLBHSA-N
Gene Information
human ... CA1(759) , CA2(760)
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as an aspartame (ASP) metabolite to determine acetylcholinesterase (AChE) activity in the hippocampal homogenate of suckling rats
- as printed chemotaxis ligands to screen chemotaxis receptors with high-throughput technologies and verify the interactions with surface plasmon resonance (SPR) and nuclear magnetic resonance (NMR)
- as a supplement in Dulbecco′s modified Eagle′s medium (DMEM) for supplementation assays of various cultured cell lines
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service