82415
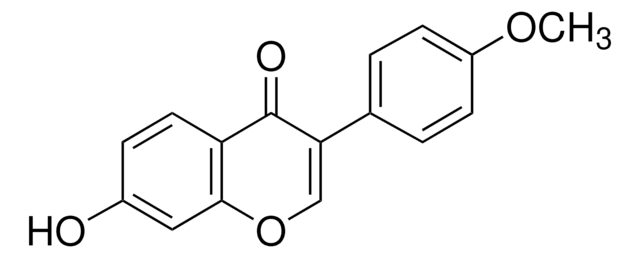
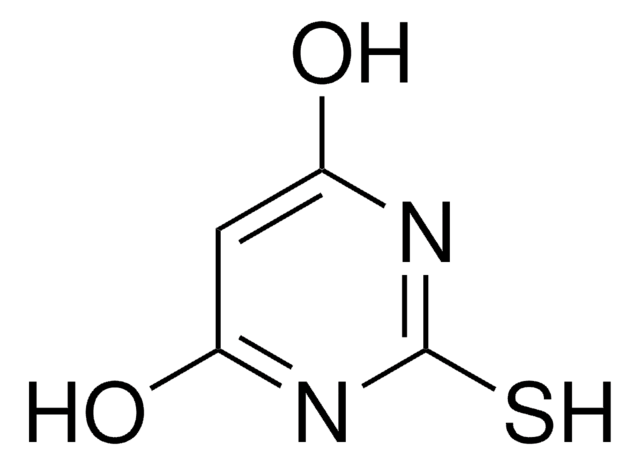
Prunetin
≥98.0% (TLC)
Synonym(s):
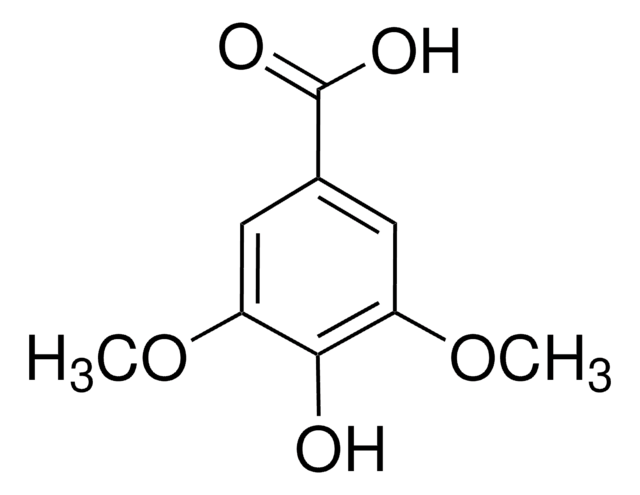
4′,5-Dihydroxy-7-methoxyisoflavone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H12O5
CAS Number:
Molecular Weight:
284.26
Beilstein:
292155
EC Number:
MDL number:
UNSPSC Code:
51111800
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98.0% (TLC)
form
powder
SMILES string
COc1cc(O)c2C(=O)C(=COc2c1)c3ccc(O)cc3
InChI
1S/C16H12O5/c1-20-11-6-13(18)15-14(7-11)21-8-12(16(15)19)9-2-4-10(17)5-3-9/h2-8,17-18H,1H3
InChI key
KQMVAGISDHMXJJ-UHFFFAOYSA-N
Biochem/physiol Actions
Inhibitor of both cytoplasmic and mitochondrial human liver aldehyde dehydrogenases, possibly by binding at an allosteric site.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Soon-Sen Leow et al.
Experimental gerontology, 106, 198-221 (2018-03-20)
Palm fruit juice (PFJ) containing oil palm phenolics is obtained as a by-product from oil palm (Elaeis guineensis) fruit milling. It contains shikimic acid, soluble fibre and various phenolic acids including p-hydroxybenzoic acid and three caffeoylshikimic acid isomers. PFJ has
M L Shen et al.
Journal of the American Society for Mass Spectrometry, 12(1), 97-104 (2001-01-06)
Aldehyde dehydrogenases (ALDH) are a family of enzymes primarily involved in the oxidation of various aldehydes. Most ALDH enzymes derived from mammalian sources have been shown to exist as homotetramers, consisting of four identical subunits of approximately 54 kDa. The
N S Nagarajan et al.
Natural product research, 20(2), 195-200 (2005-12-02)
Two isoflavonoids isolated from Dalbergia sympathetica were identified as 5,4'-dihydroxy-7-methoxyisoflavone (1) (Prunetin) and Prunetin-4'-O-beta-D-gentiobioside (2) (Dalsympathetin). The natural occurrence of Dalsympathetin is reported for the first time. The position of glycosylation in Dalsympathetin at 4'-position has been confirmed by 2D-NMR
Stephen W J Wang et al.
The Journal of pharmacology and experimental therapeutics, 329(3), 1023-1031 (2009-03-07)
Flavonoids have poor bioavailabilities largely because of metabolism via UDP-glucuronosyltransferases (UGTs). This study aims to further understand the functions of UGT in metabolizing genistein and apigenin, two compounds metabolized more extensively in the gut than in the liver. Because Gunn
Hyun Jae Lee et al.
Phytotherapy research : PTR, 25(8), 1196-1200 (2011-02-10)
This study investigated whether prunetin significantly affects the secretion, production and gene expression of mucin from cultured airway epithelial cells. Confluent primary rat tracheal surface epithelial (RTSE) cells were pretreated with adenosine triphosphate (ATP) for 5 min and then chased for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service