F7503
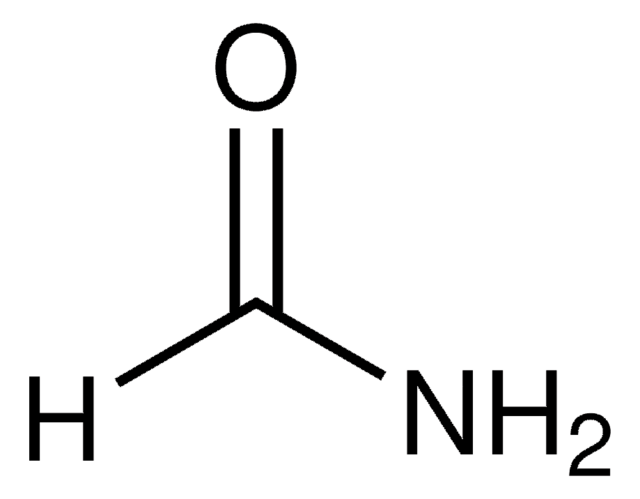
Formamide
ReagentPlus®, ≥99.0% (GC)
Synonym(s):
Amide C1, Formic amide
About This Item
Recommended Products
vapor density
1.55 (vs air)
Quality Level
vapor pressure
0.08 mmHg ( 20 °C)
30 mmHg ( 129 °C)
product line
ReagentPlus®
Assay
≥99.0% (GC)
form
liquid
autoignition temp.
932 °F
expl. lim.
2.7-19 %
technique(s)
hybridization: suitable
refractive index
n20/D 1.447 (lit.)
pH
4-10 (20 °C, 200 g/L)
bp
210 °C (lit.)
mp
2-3 °C (lit.)
density
1.134 g/mL at 25 °C (lit.)
SMILES string
NC=O
InChI
1S/CH3NO/c2-1-3/h1H,(H2,2,3)
InChI key
ZHNUHDYFZUAESO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To synthesize tetrakis(trialkylsilyl) diphosphate by reacting disodium dihydrogen diphosphate with trialkyl chlorosilane.
- In Cu/C catalyzed cycloaddition between azides and terminal alkynes to synthesize triazoles.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Repr. 1B - STOT RE 2 Oral
Target Organs
Blood
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
305.6 °F
Flash Point(C)
152 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
GC Analysis of Class 2 Residual Solvents on OVI-G43
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service