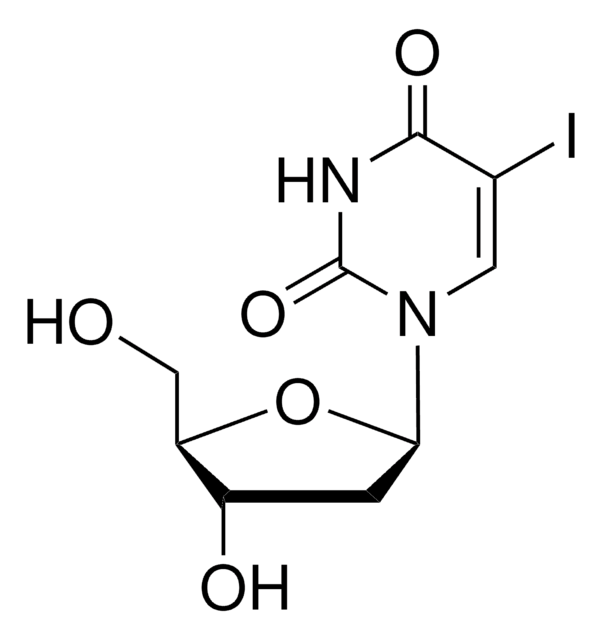
909505
C8-Alkyne-dU-CEP
Synonym(s):
5-(Octa-1,7-diynyl)-3′-O-[(2-cyanoethoxy)(diisopropylamino)-phosphono)]-5′-O- (4,4′-dimethoxytrityl)-2′-deoxyuridine, 5-Octadiynyl-dU CEP, C8-Alkyne dU phosphoramidite
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C47H55N4O8P
CAS Number:
Molecular Weight:
834.94
NACRES:
NA.22
Recommended Products
form
powder
reaction suitability
reaction type: click chemistry
storage temp.
−20°C
Application
This functional phosphoramidite is used to introduce click handles univerally (5′ internal and 3′end) into oligonucleotides under standard conditions for solid phase synthesis. Afterwards the oligonucleotide (RNA, PNA, DNA) can be efficiently conjugated using a copper-catalyzed click reaction to azide-functionalized moieties such as fluorescent dye azides and azido amino acids
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Frank Seela et al.
Chemistry & biodiversity, 3(5), 509-514 (2006-12-29)
The synthesis of a series of oligonucleotides containing 5-substituted pyrimidines as well as 7-substituted 7-deazapurines bearing diyne groups with terminal triple bonds is reported. The modified nucleosides were prepared from the corresponding iodo nucleosides and diynes by the Sonogashira cross-coupling
DNA photography: an ultrasensitive DNA-detection method based on photographic techniques.
David M Hammond et al.
Angewandte Chemie (International ed. in English), 46(22), 4184-4187 (2007-04-27)
Dorota I Rozkiewicz et al.
Chembiochem : a European journal of chemical biology, 8(16), 1997-2002 (2007-09-15)
This paper describes a straightforward procedure to immobilize oligonucleotides on glass substrates in well-defined micropatterns by microcontact printing with a dendrimer-modified stamp. The oligonucleotides are efficiently immobilized by "click" chemistry induced by microcontact printing. Acetylene-modified oligonucleotides were treated with an
Johannes Gierlich et al.
Organic letters, 8(17), 3639-3642 (2006-08-11)
[reaction: see text] We report the development of the Cu(I)-catalyzed Huisgen cycloaddition (click) reaction for the multiple postsynthetic labeling of alkyne-modified DNA. A series of alkyne-modified oligodeoxyribonucleotides (ODNs) of increasing alkyne density were prepared, and the click reaction using various
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)

![3-Bromo-1H-pyrazolo[3,4-d]pyrimidin-4-amine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/794/777/0edc2115-9a26-4d94-8c55-80a2d58bdae7/640/0edc2115-9a26-4d94-8c55-80a2d58bdae7.png)
