10110434001
Roche
Xanthine Oxidase (XOD)
from cow milk
Synonym(s):
XOD
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
bovine milk
Quality Level
form
suspension
specific activity
~1 units/mg protein (At 25 °C with xanthine as the substrate.)
packaging
pkg of 1 mL (20 U)
manufacturer/tradename
Roche
optimum pH
8.5-9.0
shipped in
wet ice
storage temp.
2-8°C
General description
Xanthine Oxidase (XOD) is a metal flavoprotein. It has FAD, molybdenum and iron in the ratio 2:2:8.
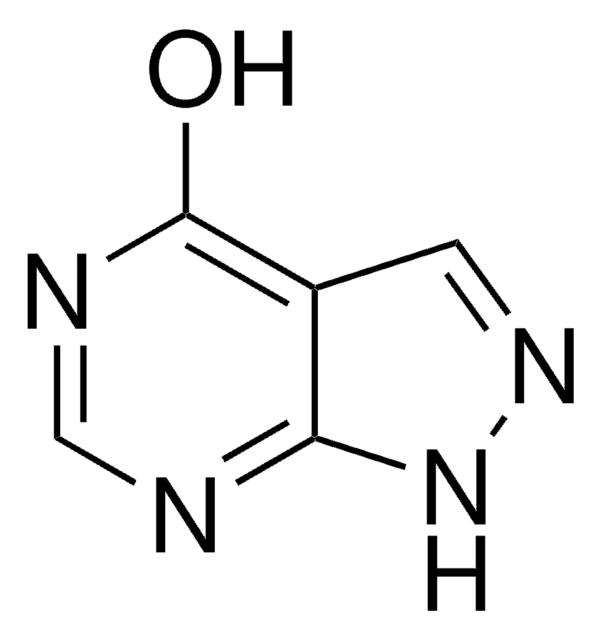
Xanthine:oxygen oxidoreductase
Application
Xanthine Oxidase (XOD) has been used in the assessment of XOR-mediated NO production from NDHP and assessment of nitrite-derived NO in liver and purified XOR.
Xanthine Oxidase has been used to study tyrosine nitration.
Biochem/physiol Actions
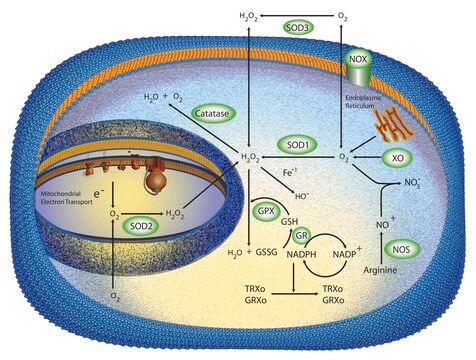
Xanthine Oxidase (XOD) exhibits a broad substrate specificity including aldehydes, purines and pteridines. Furthermore, this enzyme reduces oxygen to generate superoxide, hydrogen peroxide and reactive oxygen species (ROS). It also reduces nitrite to yield reactive nitrogen species (RNS), such as peroxynitrite and nitric oxide. Owing to its ability to generate RNS and ROS, XOD might play an important role as an antimicrobial agent in the neonatal gut, thereby complementing endogenous enzyme of the intestinal epithelium.
Quality
Contaminants: <0.005% guanase, NP and uricase, each, <0.05% ADA, <0.05% alkaline phosphatase (4-nitrophenyl phosphate as the substrate)
Note: Chromatographically purified.
Note: Chromatographically purified.
Sequence
XOD is a dimer. Each subunit contains 1 atom of molybdenum, 2 iron-sulfur centers (non-heme iron, ferredoxin-type) and 1 molecule of FAD.
Unit Definition
One unit (U) xanthine oxidase will produce 1 μmol of uric acid (E293nm = 12.2 mmol -1 x L x cm-1) from the oxidation of 1 μmol of xanthine in 1 min at 25 °C and pH8.5.
Physical form
Suspension in 3.2 M ammonium sulfate solution, 10 mM EDTA, pH approximately 8
Preparation Note
Activator: O2
Stabilizers: The substance is stabilized by the addition of EDTA; salicylate is not added.
Stabilizers: The substance is stabilized by the addition of EDTA; salicylate is not added.
Other Notes
For life science research only. Not for use in diagnostic procedures.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
does not flash
Flash Point(C)
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Sawa et al.
The Journal of biological chemistry, 275(42), 32467-32474 (2000-07-25)
Peroxynitrite (ONOO(-)) is a potent nitrating and oxidizing agent that is formed by a rapid reaction of nitric oxide (NO) with superoxide anion (O(2)). It appears to be involved in the pathophysiology of many inflammatory and neurodegenerative diseases. It has
Methods of Enzymatic Analysis (2012)
The novel organic mononitrate NDHP attenuates hypertension and endothelial dysfunction in hypertensive rats.
Paulo LL, et al.
Redox Biology, 15, 182-191 (2018)
International Dairy Journal
R. Harrison.
Science, 16 (6), 546-554 (2006)
Synthesis and characterization of a novel organic nitrate NDHP: Role of xanthine oxidoreductase-mediated nitric oxide formation.
Zhuge Z, et al.
Redox Biology, 13, 163-169 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service