TR-1003
Polybrene Infection / Transfection Reagent
A highly efficient method of gene transfer into mammalian cells leveraging infection with retroviral vectors.
Synonym(s):
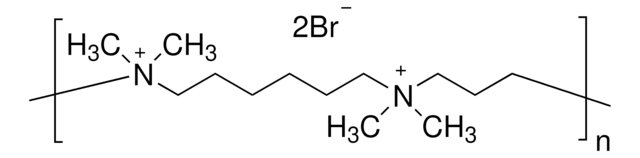
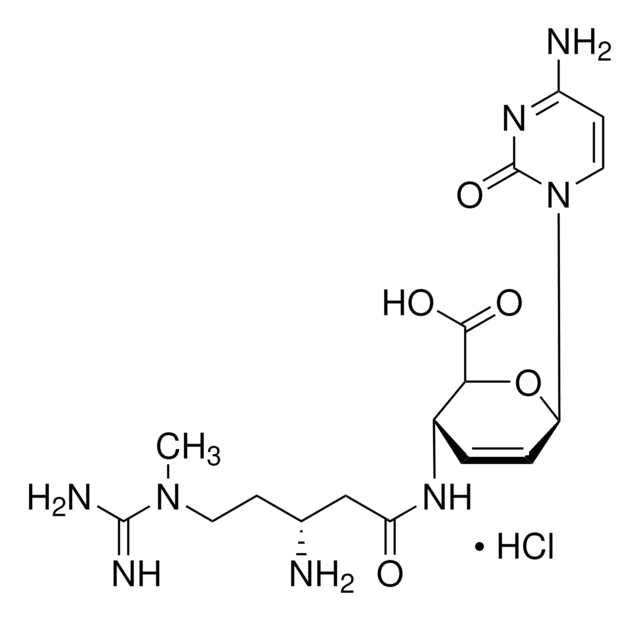
1,5-Dimethyl-1,5-diazaundecamethylene polymethobromide, hexamethrine bromide, retroviral infection
About This Item
Recommended Products
Application
Protocol: Retroviral Infection
Recombinant retroviral stocks are prepared by adding 5mls of growth medium with 5% serum to a near confluent monolayer of transfected retroviral packaging cells in a 100mm plate. After 24 hours the medium is removed and filtered through a 0.45um filter.
Cells to be infected with this recombinant retroviral stock are plated at 500,000 cells per 100mm plate in 10mls of complete medium.
24 hours later, remove the growth medium from the cells. Infect cells with 2mls of the viral supernatant (or a dilution of the virus stock into 2mls) in the presence of 5ug to 10ug of polybrene per ml (final concentration). Incubate cells for 3 to 6 hours at 37°C.
Add 8mls of complete medium. Three days after infection, split the cells 1:5 into selection medium.
Protocol: Transfection
Plate cells at approximately 50% confluence in complete growth medium.
18 to 24 hours post plating, prepare the DNA-Medium-Polybrene solution, immediately before using as follows:
Note: Each component must be added in the proper sequence.
1st: Complete growth medium (2mls for a 60mm plate and 3mls for
a 100mm plate) warmed to 37°C.
2nd: Plasmid DNA, 10ng to 10ug. Gently mix.
3rd: Polybrene to a final concentration of 5ug to 10ug per ml. Gently mix
Remove medium from plate and add DNA-Medium-Polybrene solution to cells. Incubate cells at 37°C for 6 to 20 hours with occasional gentle rocking approximately every 1.5 hours for the first 6 hours.
Remove DNA-Medium-Polybrene solution and gently overlay cells with DMSO shock solution (15% DMSO in 1X HBSS: Specialty Media catalog #S-051-D) 3mls per 60mm dish and 4mls per 100mm plate. Manually rock the dish for 10 seconds to evenly distribute the solution, and then incubate the cells for exactly 4 minutes at 37°C.
Immediately remove the DMSO shock solution and gently rinse the cells twice with complete growth medium, 5mls per wash per 60mm dish, 10mls per wash per 100mm dish
Add complete growth medium to the cells.
For Stable transformants, remove the growth media and split the cells 1:5 into selection medium.
For transient expression, remove the growth medium and add fresh growth medium. Harvest cells and/or medium after 24 to 72 hours.
Reagent is supplied filtered through 0.2μm membranes and hydrated with sterile H2O.
Physical form
Storage and Stability
Disclaimer
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
High titer lentiviral particles for LC3 variants used for live cell analysis of cellular autophagy.
High titer lentiviral particles including beta-actin, alpha-tubulin and vimentin used for live cell analysis of cytoskeleton structure proteins.
Protocols
Stem cell reprogramming protocols to generate human induced pluripotent stem cells (iPSCs) including viral and non-viral RNA based methods.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service