W322601
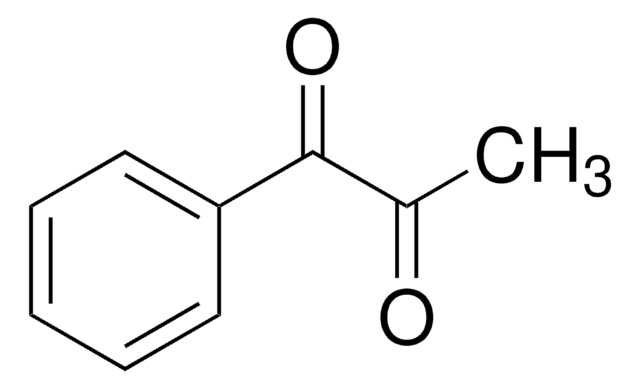
1-Phenyl-1,2-propanedione
98%, FG
Synonym(s):
Acetyl benzoyl
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
Assay
98%
refractive index
n20/D 1.532 (lit.)
bp
103-105 °C/14 mmHg (lit.)
density
1.101 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
honey; buttery; plastic
SMILES string
CC(=O)C(=O)c1ccccc1
InChI
1S/C9H8O2/c1-7(10)9(11)8-5-3-2-4-6-8/h2-6H,1H3
InChI key
BVQVLAIMHVDZEL-UHFFFAOYSA-N
Gene Information
human ... ACHE(43) , BCHE(590) , CES1(1066)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A Simplified Kinetic Model for the Enantioselective Hydrogenation of 1-Phenyl-1,2-Propanedione over Ir/TiO(2) in the Presence of a Chiral Additive.: This study presents a simplified kinetic model for the enantioselective hydrogenation of 1-Phenyl-1,2-propanedione, emphasizing its potential in synthetic organic chemistry and catalysis, which could have significant implications for pharmaceutical synthesis and industrial applications (Melián-Cabrera et al., 2022).
- Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts.: Although primarily focused on photoinitiating systems for dental materials, this review could indirectly encompass applications of 1-Phenyl-1,2-propanedione in dental photopolymerization processes, suggesting potential for further exploration in dental material enhancements (Topa and Ortyl, 2020).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
183.2 °F - closed cup
Flash Point(C)
84 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service