All Photos(1)
About This Item
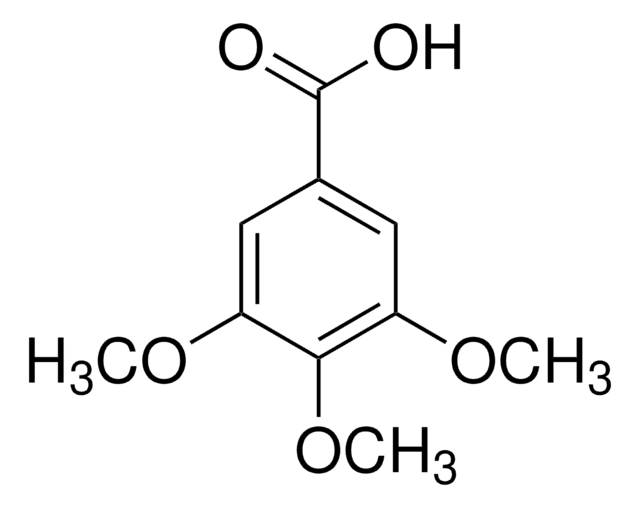
Linear Formula:
(CH3O)3C6H2CH2CO2H
CAS Number:
Molecular Weight:
226.23
Beilstein:
2697844
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
117-120 °C (lit.)
SMILES string
COc1cc(CC(O)=O)cc(OC)c1OC
InChI
1S/C11H14O5/c1-14-8-4-7(6-10(12)13)5-9(15-2)11(8)16-3/h4-5H,6H2,1-3H3,(H,12,13)
InChI key
DDSJXCGGOXKGSJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ahmet Şenocak et al.
Dalton transactions (Cambridge, England : 2003), 47(29), 9617-9626 (2018-07-04)
The synthesis and characterization of new hybrid materials based on reduced graphene oxide (rGO) or single walled carbon nanotubes (SWCNTs) covalently functionalized by 4,4'-difluoro-8-(4-propynyloxy)-phenyl-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene (BODIPY) (2) or 7-(prop-2-yn-1-yloxy)-3-(3',4',5'-trimethoxyphenyl)-coumarin (4) as light harvesting groups have been described. The organic solar cell
M I Donnelly et al.
Journal of bacteriology, 147(2), 477-481 (1981-08-01)
When grown at the expense of 3,4,5-trimethoxyphenylacetic acid, a species of Arthrobacter readily oxidized 3,4-dihydroxy-5-methoxyphenylacetic acid, but other structurally related aromatic acids were oxidized only slowly. Cell extracts contained a dioxygenase for 3,4-dihydroxy-5-methoxyphenylacetate, and the corresponding trihydroxy acid, which was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service