P6513
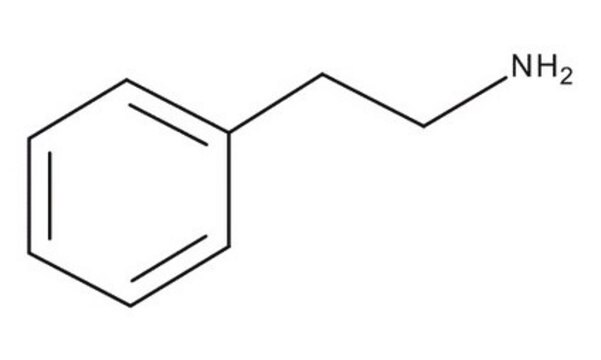
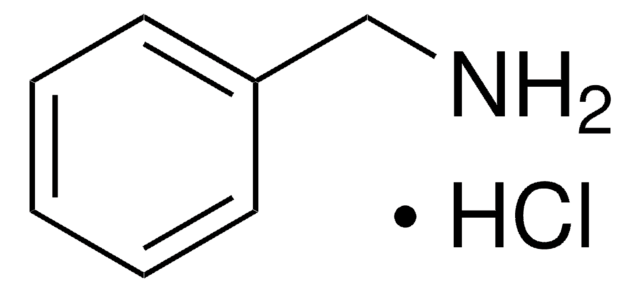
2-Phenylethylamine hydrochloride
≥98%
Synonym(s):
β-Phenylethylamine hydrochloride, Phenethylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
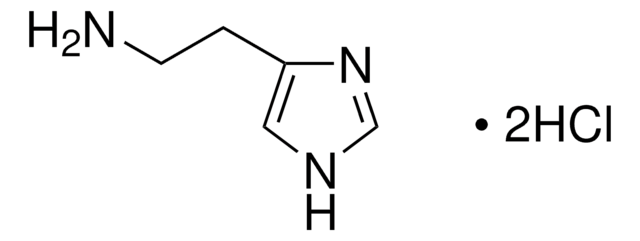
C6H5CH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
157.64
Beilstein:
3624163
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
powder
mp
220-222 °C (lit.)
SMILES string
Cl.NCCc1ccccc1
InChI
1S/C8H11N.ClH/c9-7-6-8-4-2-1-3-5-8;/h1-5H,6-7,9H2;1H
InChI key
SKHIBNDAFWIOPB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Phenylethylamine hydrochloride is a biogenic aromatic amine.
Application
- Multifunctional organic electrolyte additive for batteries: Research outlined a novel application of 2-Phenylethylamine hydrochloride in developing a multifunctional organic electrolyte additive for aqueous zinc ion batteries, enhancing the performance of polyaniline cathodes (Wang et al., 2023).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Simultaneous analysis of biogenic amines in canned fish by HPLC.
Yen GC & Hsieh CL
Journal of Food Science, 56(1), 158-160 (1991)
Calix [5] arene-based heteroditopic receptor for 2-phenylethylamine hydrochloride
Gargiulli C, et al.
The Journal of Organic Chemistry, 74(11), 4350-4353 (2009)
Elena Cichero et al.
Chemical biology & drug design, 81(4), 509-516 (2012-08-14)
Trace amine-associated receptor 1 (TAAR1) is a G protein-coupled receptor that belongs to the family of TAAR receptors and responds to a class of compounds called trace amines, such as β-phenylethylamine (β-PEA) and 3-iodothyronamine (T(1)AM). The receptor is known to
Christian Brand et al.
The journal of physical chemistry. A, 115(34), 9612-9619 (2011-04-20)
A remarkable influence of the orientation of a polar side chain on the direction of the S(1) ← S(0) transition dipole moment of monosubstituted benzenes was previously reported from high-resolution electronic spectroscopy. In search for a more general understanding of
Shinsuke Inagaki et al.
Drug testing and analysis, 4(12), 1001-1008 (2012-03-13)
A rapid enantiomeric separation and simultaneous determination method based on ultra high performance liquid chromatography (UHPLC) was developed for phenethylamine-type abused drugs using (R)-(-)-4-(N,N-dimethylaminosulfonyl)-7-(3-isothiocyanatopyrrolidin-1-yl)-2,1,3-benzoxadiazole ((R)-(-)-DBD-Py-NCS) as the chiral fluorescent derivatization reagent. The derivatives were rapidly enantiomerically separated by reversed-phase UHPLC
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service