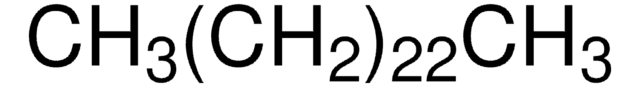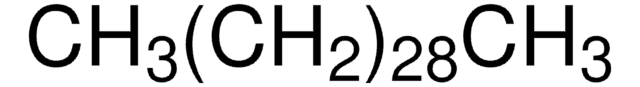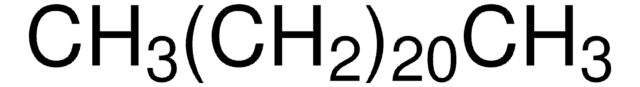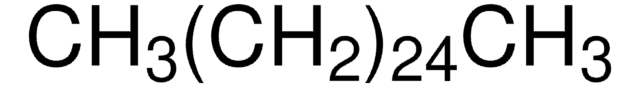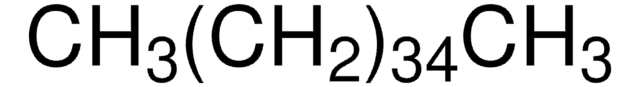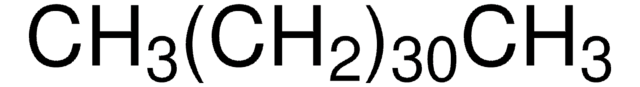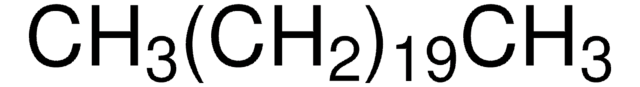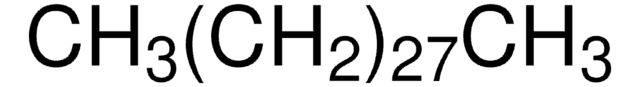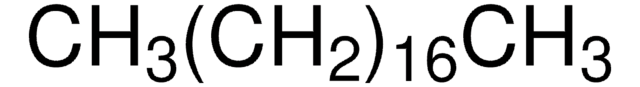About This Item
Recommended Products
vapor density
13.6 (vs air)
vapor pressure
<1 mmHg ( 20 °C)
Assay
99%
form
powder
bp
278 °C/15 mmHg (lit.)
mp
57-64 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCCCCCCCCCCCCC
InChI
1S/C28H58/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h3-28H2,1-2H3
InChI key
ZYURHZPYMFLWSH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
440.6 °F
Flash Point(C)
227 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Decane; Dodecane; Tetradecane; Hexadecane; Octadecane; Eicosane; Docosane; Tetracosane; Hexacosane; Octacosane
Separation of Hexane; Heptane; Octane; Nonane; Decane; Undecane; Dodecane; Tetradecane; Hexadecane; Octadecane; Eicosane; Tetracosane; Octacosane; Dotriacontane; Hexatriacontane; Tetracontane; Tetratetracontane
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service