H41803
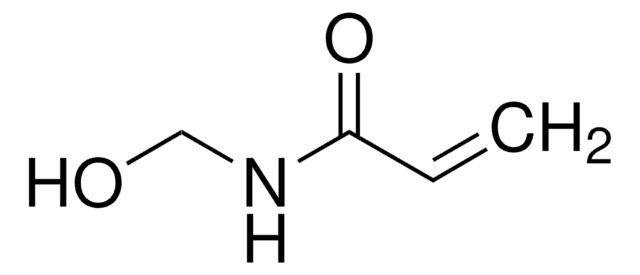
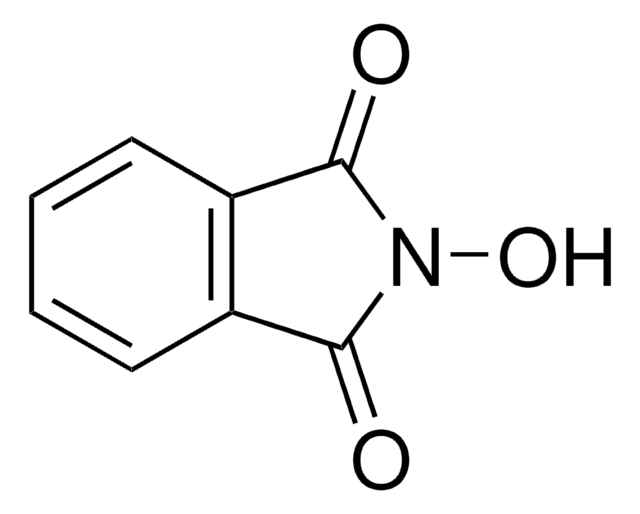
N-(Hydroxymethyl)phthalimide
97%
Synonym(s):
Phthalimidomethanol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H7NO3
CAS Number:
Molecular Weight:
177.16
Beilstein:
140946
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
147-149 °C (lit.)
SMILES string
OCN1C(=O)c2ccccc2C1=O
InChI
1S/C9H7NO3/c11-5-10-8(12)6-3-1-2-4-7(6)9(10)13/h1-4,11H,5H2
InChI key
MNSGOOCAMMSKGI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
For amidomethylation of aromatics in triflic acid.
Packaging
Safe reagent for the in situ generation of anhydrous formaldehyde in organic solvents.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, 1077-1077 (1993)
[Effect of hydroxymethylphthalimide on embryogenesis in white rats].
Iu S Kagan et al.
Gigiena i sanitariia, (10)(10), 64-66 (1983-10-01)
Organic Letters (2007)
M N Khan
Journal of pharmaceutical and biomedical analysis, 7(6), 685-691 (1989-01-01)
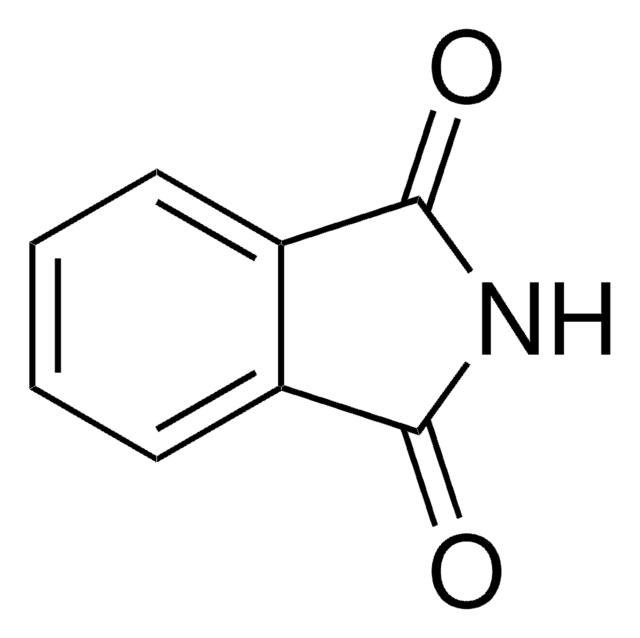
The conversion of N-(hydroxymethyl)phthalimide (NHPH) to phthalimide could not be detected within 300 s at pH 9.0, whereas in 0.18 M NaOH complete conversion of NHPH to phthalimide was observed within 50 s. In the presence of 0.2-0.4 M 1,4-diazabicyclo[2.2.2]octane
Xiang-Bao Meng et al.
Carbohydrate research, 342(9), 1169-1174 (2007-04-05)
3,4,6-Tri-O-acetyl-D-galactal, 3,4,6-tri-O-acetyl-D-glucal and 3,6,2',3',4'6'-hexa-O-acetyl-D-lactal were reacted with N-hydroxymethylphthalimide and boron trifluoride etherate to produce the corresponding phthalimidomethyl unsaturated glycosides via Ferrier rearrangement. When the galactal derivative was used, a non-Ferrier rearrangement product was also isolated as a minor product under
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)