914584
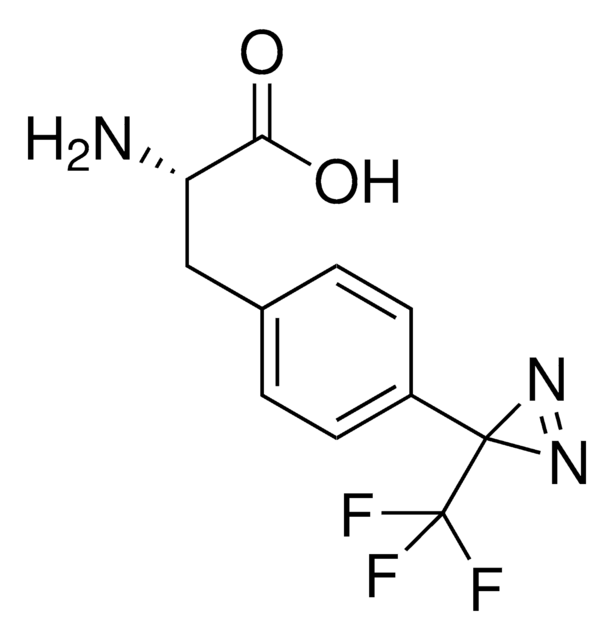
Phenylalanine-4′-azobenzene HCl
≥95%
Synonym(s):
(2S)-2-Amino-3-[4-(2-phenyldiazen-1-yl)phenyl]propanoic acid hydrochloride, (E)-2-Amino-3-(4-(phenyldiazenyl)phenyl)propanoic acid HCl, 4-(2-Phenyldiazenyl)-L-phenylalanine hydrochloride, 4-(Phenylazo)-L-phenylalanine, AzoF, Photoswitchable phenylalanine amino acid, Reversible photo-controllable unnatural amino acid
About This Item
Recommended Products
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Photochemical control of enzymes using photoresponsive compounds on the basis of the interaction mechanism
Photochemical regulation of the activity of an endonuclease BamHI using an azobenzene moiety incorporated site-selectively into the dimer interface
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![5-Amino-1H-[1,2,4]-triazole-3-carboxylic acid methyl ester 96%](/deepweb/assets/sigmaaldrich/product/structures/343/124/fed9a51c-601d-495f-8ded-bd63502da7ca/640/fed9a51c-601d-495f-8ded-bd63502da7ca.png)