913030
trYPhos™
Umicore
Synonym(s):
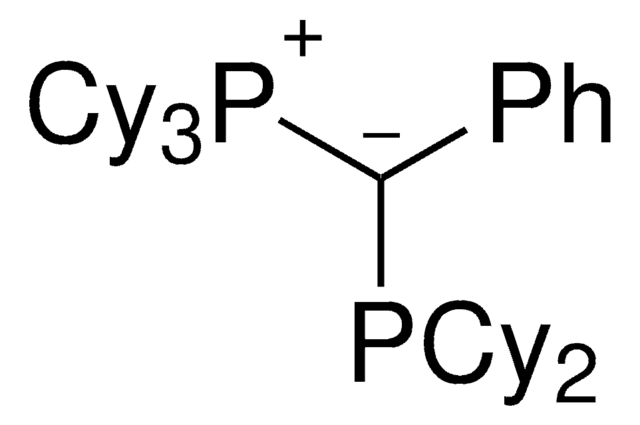
(CyYPhos)(Me)PtBu2, Tricyclohexyl(1-(di-tert-butylphosphanyl)ethylidene)phosphane
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C28H54P2
CAS Number:
Molecular Weight:
452.68
UNSPSC Code:
12352001
NACRES:
NA.22
Recommended Products
Quality Level
form
powder
reaction suitability
reagent type: ligand
mp
137-139 °C
functional group
phosphine
General description
trYPhos™ is an ylide-functionalized phosphine ligand developed in the lab of Prof. V. Gessner with demonstrated uses in Pd-catalyzed cross coupling reactions, including the arylation of ketones and arylation of amines. trYPhos™is the strongest donor of the available YPhos™ ligands, and is suited towards the most challenging alpha-arylation reactions.
Application
The electron-rich and sterically demanding trYPhos™ ligand has been used in the gold(I)-catalyzed hydroamination of acetylene, and has shown to be effective in a range of Buchwald-Hartwig amination reactions. The strong electron-donor strength and sterically demanding nature of the ligand has been shown to increase the rate of formation of the catalytically active mono-phosphine palladium species, often leading to decreased reaction times or allowing the use of lower reaction temperatures. trYPhos™ is the most strongest donor of the available YPhos™ligands and has been found to be effective alpha-arylation of aliphatic cyclic ketones.
Learn more about ylide-functionalized phosphines (YPhos)
Learn more about ylide-functionalized phosphines (YPhos)
Features and Benefits
Advantages of the trYPhos™ ligand over less electron rich ligand sources include, increased substrate scope in Buchwald-Hartwig amination reactions, including aryl chlorides, the use of more mild reaction conditions and improved activity in in C-N and C-C cross coupling reactions. The increased electron donor stregth of trYPhos™ has been shown to be effective in the challenging alpha-arylation of aliphatic ketones.
Legal Information
Product of Umicore
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
Yphos is a trademark of Umicore AG & Co. KG
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jens Tappen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(19), 4281-4288 (2020-01-24)
Palladium allyl, cinnamyl, and indenyl complexes with the ylide-substituted phosphines Cy3 P+ -C- (R)PCy2 (with R=Me (L1) or Ph (L2)) and Cy3 P+ -C- (Me)PtBu2 (L3) were prepared and applied as defined precatalysts in C-N coupling reactions. The complexes are
Xiao-Qiang Hu et al.
Organic letters, 21(18), 7558-7562 (2019-08-31)
Ylide-functionalized phosphine (YPhos) ligands allow the palladium-catalyzed α-arylation of alkyl ketones with aryl chlorides with record setting activity. Using a cyclohexyl-substituted YPhos ligand, a wide range of challenging ketone substrates was efficiently and selectively monoarylated under mild conditions. A newly
Ilja Rodstein et al.
The Journal of organic chemistry, 85(22), 14674-14683 (2020-09-11)
Ylide-substituted phosphines have been shown to be excellent ligands for C-N coupling reactions under mild reaction conditions. Here we report studies on the impact of the steric demand of the substituent in the ylide-backbone on the catalytic activity. Two new
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service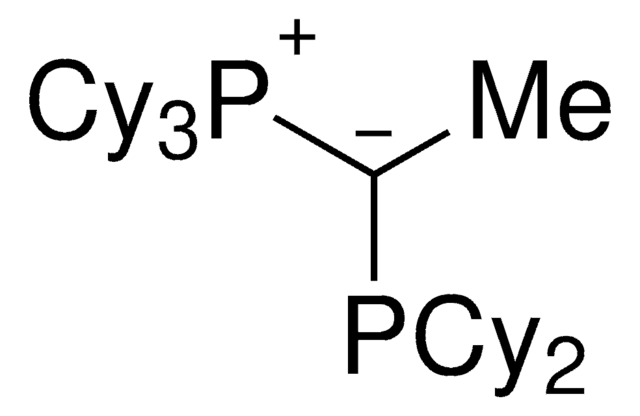
![[Pd(IPr#)(cin)Cl]](/deepweb/assets/sigmaaldrich/product/structures/391/578/9bb7eaef-fa70-4f50-8644-2c55cec3925d/640/9bb7eaef-fa70-4f50-8644-2c55cec3925d.png)