900814
SLAP TM
≥95%
Synonym(s):
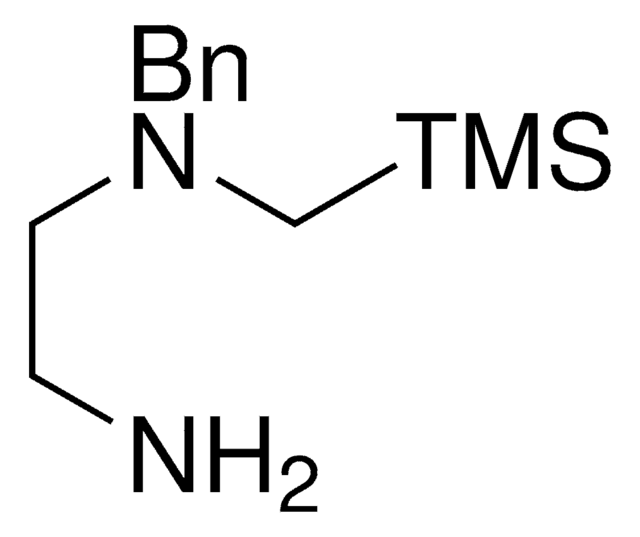
2-(((Trimethylsilyl)methyl)thio)ethanamine
About This Item
Recommended Products
Assay
≥95%
form
liquid
refractive index
n/D 1.4811
density
0.90746 g/mL
storage temp.
2-8°C
SMILES string
NCCSC[Si](C)(C)C
Application
Other Notes
- Technology Spotlight: SLAP Reagents for Piperazine Synthesis
- Silicon Amine Reagents for the Photocatalytic Synthesis of Piperazines from Aldehydes and Ketones
- Lewis Acid Induced Toggle from Ir(II) to Ir(IV) Pathways in Photocatalytic Reactions: Synthesis of Thiomorpholines and Thiazepanes from Aldehydes and SLAP Reagents.
- Continuous Flow Synthesis of Morpholines and Oxazepanes with Silicon Amine Protocol (SLAP) Reagents and Lewis Acid Facilitated Photoredox Catalysis
related product
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.0 °F
Flash Point(C)
78.89 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
The Bode group has developed SnAP (stannyl amine protocol) reagents that cross-couple with aldehydes and ketones to provide one-step access to a wide variety of saturated N-heterocycles.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service