All Photos(2)
About This Item
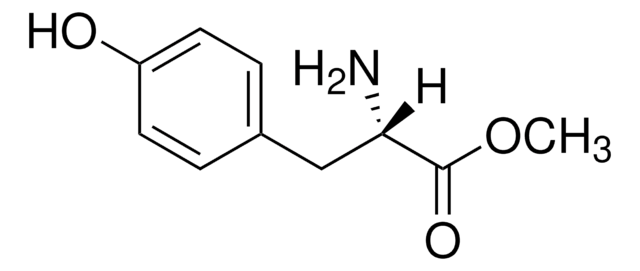
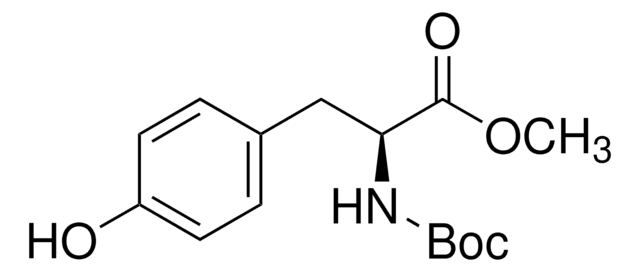
Linear Formula:
HOC6H4CH2CH(NH2)COOCH3 · HCl
CAS Number:
Molecular Weight:
231.68
Beilstein:
3917353
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
optical activity
[α]25/D +74.0°, c = 3 in pyridine
reaction suitability
reaction type: solution phase peptide synthesis
mp
192 °C (dec.) (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
Cl.COC(=O)[C@@H](N)Cc1ccc(O)cc1
InChI
1S/C10H13NO3.ClH/c1-14-10(13)9(11)6-7-2-4-8(12)5-3-7;/h2-5,9,12H,6,11H2,1H3;1H/t9-;/m0./s1
InChI key
VXYFARNRGZWHTJ-FVGYRXGTSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Francesca Fontana et al.
Molecules (Basel, Switzerland), 26(2) (2021-01-14)
Configurationally stable 5-aza[6]helicene (1) was envisaged as a promising scaffold for non-conventional ionic liquids (IL)s. It was prepared, purified, and separated into enantiomers by preparative HPLC on a chiral stationary phase. Enantiomerically pure quaternary salts of 1 with appropriate counterions
S Criado et al.
Photochemistry and photobiology, 68(4), 453-458 (1998-10-31)
This paper studies the dye-sensitized photooxidation of tyrosine (tyr) and tyr di- and tripeptides (tyr-tyr and tyr-tyr-tyr) mediated by singlet molecular oxygen (O2[1 delta g]) in alkaline media. Photooxidation quantum efficiencies (phi r) were obtained by determining the overall and
J Brtko et al.
Molecular and cellular endocrinology, 93(1), 81-86 (1993-05-01)
Various protease inhibitors (e.g. phenylmethanesulfonyl fluoride (PMSF), tosyl-phenylalanine chloromethyl ketone (TosPheCH2Cl)) and substrates (e.g., tosyl-arginine methyl ester (TosArgOMe), tryptophan methyl ester (TrpOMe)) inhibit the binding of adrenal and sex steroids to their cognate receptors (Hubbard and Kalimi (1985) Mol. Cell.
János Elek et al.
Journal of pharmaceutical and biomedical analysis, 38(4), 601-608 (2005-06-22)
Different dual selector systems containing a cyclodextrin derivative (methyl-beta-cyclodextrin and dimethyl-beta-cyclodextrin) and a new diaza-crown-ether derivative (N-[2-(1,4,10,13-tetraoxa-7,16-diazacyclooctadecan-7-yl)propanoyl]glycine) were studied in the enantioselective separation of tryptophan-methylester and tyrosine-methylester enantiomers. This paper deals with the systematic study of the effects of changing
H Iishi et al.
International journal of cancer, 73(1), 113-116 (1997-10-23)
The effects of combined administration of a catecholamine precursor, tyrosine methyl ester (TME), and an ornithine decarboxylase (ODC) inhibitor, 1,3-diaminopropane (DAP), on the incidence of gastric cancers induced by N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), the norepinephrine (NE) concentration and ODC activity of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service