765112
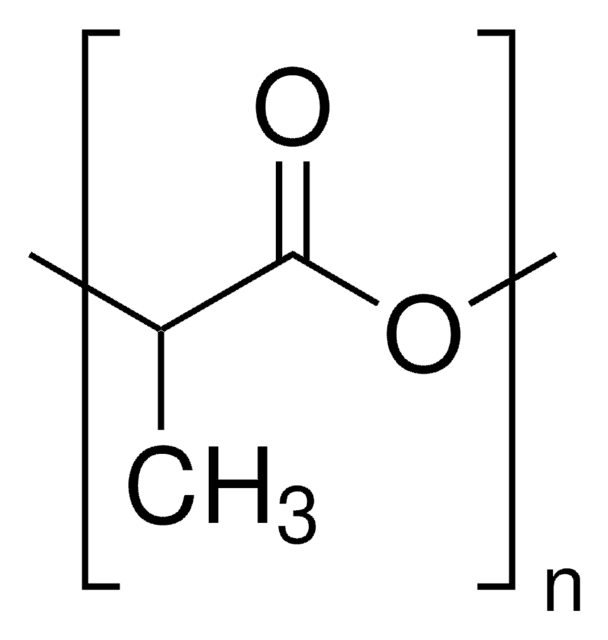
Poly(L-lactide)
average Mn 10,000, PDI ≤1.1
Synonym(s):
PLA, PLLA, Polylactide, L-Lactide polymer, PLA, Poly(L-Lactic acid)
About This Item
Recommended Products
form
solid
Quality Level
optical activity
[α]22/D -150°, c = 0.5% in chloroform
mol wt
average Mn 10,000
degradation timeframe
>3 years
transition temp
Tm 162-167 °C
PDI
≤1.1
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Aran (Claremont University, USA) thoroughly discusses the engineering of graphene based materials through careful functionalization of graphene oxide, a solution processable form of graphene.
Local delivery of bioactive molecules using an implantable device can decrease the amount of drug dose required as well as non-target site toxicities compared to oral or systemic drug administration.
The world of commercial biomaterials has stagnated over the past 30 years as few materials have successfully transitioned from the bench to clinical use. Synthetic aliphatic polyesters have continued to dominate the field of resorbable biomaterials due to their long history and track record of approval with the U.S. Food and Drug Administration (FDA).
Aliphatic polyesters such as polylactide, poly(lactide-co-glycolide) and polycaprolactone, as well as their copolymers, represent a diverse family of synthetic biodegradable polymers that have been widely explored for medical uses and are commercially available.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 765112-5G | 4061832923277 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service