755745
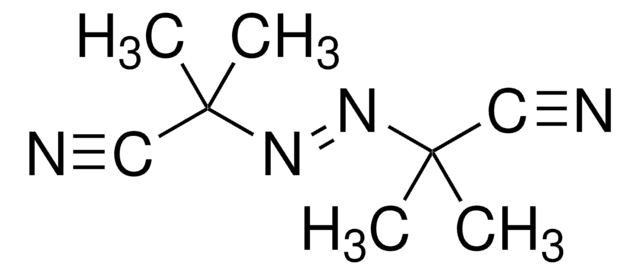
2,2′-Azobis(2-methylpropionitrile)
recrystallized from methanol, 99%
Synonym(s):
α,α′-Azoisobutyronitrile, AIBN, Azobisisobutyronitrile, Free radical initiator
About This Item
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
102-104 °C (dec.) (lit.)
103-107 °C
storage temp.
−20°C
SMILES string
CC(C)(\N=N\C(C)(C)C#N)C#N
InChI
1S/C8H12N4/c1-7(2,5-9)11-12-8(3,4)6-10/h1-4H3/b12-11+
InChI key
OZAIFHULBGXAKX-VAWYXSNFSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Porous Acid-Base Hybrid Polymers for Enhanced NH3 Uptake: This study discusses the use of 2,2′-Azobis(2-methylpropionitrile) in the synthesis of acid-base hybrid polymers, highlighting its role in enhancing ammonia uptake through cooperative hydrogen bonds (X Luo, Y Liu, et al., 2023).
- Extraction of Fluoroquinolones from Milk: The development of molecularly imprinted polymers using 2,2′-Azobis(2-methylpropionitrile) as an initiator for the extraction of antibiotics from milk showcases its application in food safety and pharmaceutical analysis (E Megias-Pérez, et al., 2023).
- Thermo-responsive Copolymer Visible Light Catalyst: Highlighting the use of 2,2′-Azobis(2-methylpropionitrile) in the synthesis of thermo-responsive copolymers, this study explores its applications in catalysis and material science, particularly in photoreactive polymers (S Wu, et al., 2024).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Self-react. C
Supplementary Hazards
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flash Point(F)
122.0 °F
Flash Point(C)
50 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service