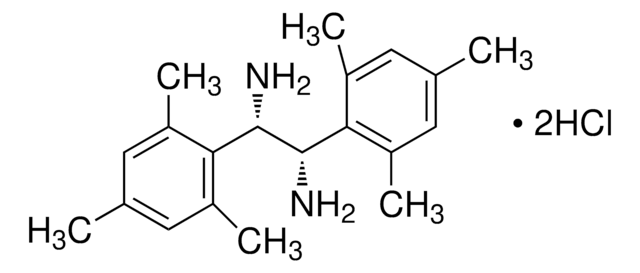
684147
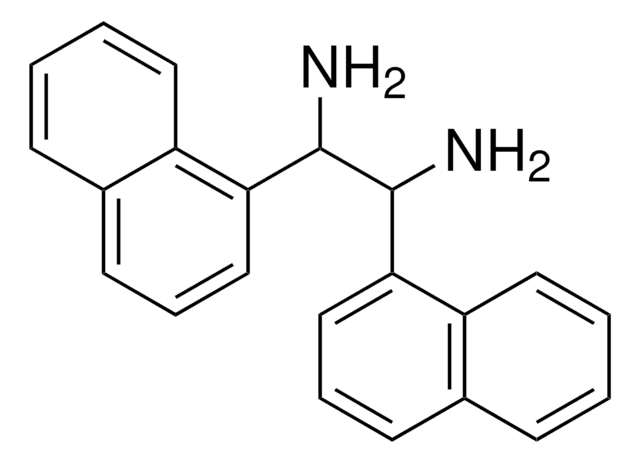
(1S, 2S)-1,2-di-1-Naphthyl-ethylenediamine dihydrochloride
97%
Synonym(s):
(S, S)-1,2-Bis(1-naphthyl)-1,2-ethanediamine dihydrochloride
About This Item
Recommended Products
Quality Level
Assay
97%
form
powder
optical activity
[α]22/D +258.0°, c = 1 in H2O
mp
219-224 °C
SMILES string
Cl[H].Cl[H].N[C@H]([C@@H](N)c1cccc2ccccc12)c3cccc4ccccc34
InChI
1S/C22H20N2.2ClH/c23-21(19-13-5-9-15-7-1-3-11-17(15)19)22(24)20-14-6-10-16-8-2-4-12-18(16)20;;/h1-14,21-22H,23-24H2;2*1H/t21-,22-;;/m0../s1
InChI key
SHNGCXWOHADIKG-IXOXMDGESA-N
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Chiral vicinal diamines are of tremendous interest to the synthetic chemist as they are found in many chiral catalysts and pharmaceuticals.
Related Content
The Chin group is interested in computational and experimental approaches to understanding stereoselective recognition and catalysis. Their studies in weak forces (H-bonding, electronic and steric effects) has led to a highly efficient method for making limitless varieties of chiral vicinal diamines from the 'mother diamine' that are useful for developing stereoselective organocatalysts or transition metal-based catalysts as well as for developing drugs (Acc Chem Res (2012) p1345). The 'mother diamine' is also useful for making binol, monophos and binap analogs. The Chin group is also interested in using reversible covalent bonds for stereoselective recognition and L to D conversion of natural and non-natural amino acids (EJOC (2012) p229).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service