480819
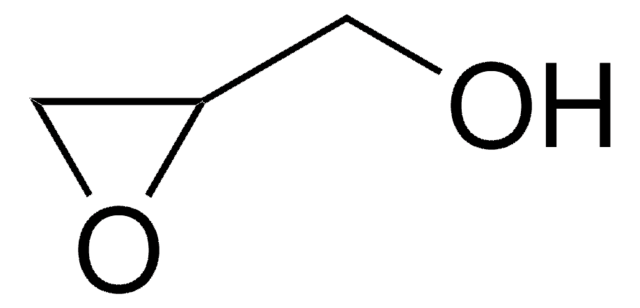
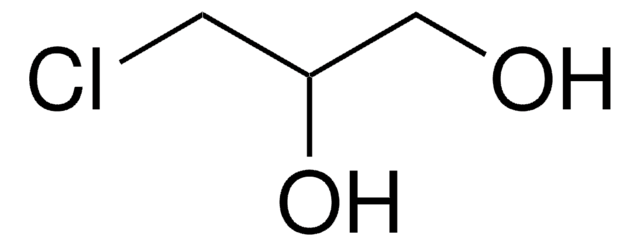
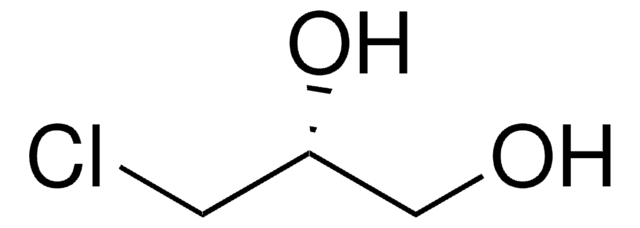
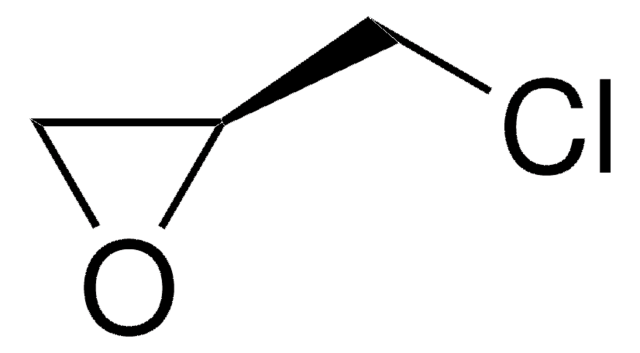
(R)-(+)-Glycidol
97%, optical purity ee: 98% (GLC)
Synonym(s):
(R)-(+)-Oxirane-2-methanol
About This Item
Recommended Products
Assay
97%
optical activity
[α]23/D +15°, neat
optical purity
ee: 98% (GLC)
impurities
<3% dichloromethane
refractive index
n20/D 1.43 (lit.)
bp
56-57 °C/11 mmHg (lit.)
density
1.116 g/mL at 20 °C (lit.)
storage temp.
−20°C
SMILES string
OC[C@@H]1CO1
InChI
1S/C3H6O2/c4-1-3-2-5-3/h3-4H,1-2H2/t3-/m1/s1
InChI key
CTKINSOISVBQLD-GSVOUGTGSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- heteroarylpropane-2,3-diols, useful for combinatorial oligomer synthesis
- enantiomerically pure (2S,3S)- and (2S,3R)-threoninol and -hydroxyphenylalaninol
- 2-amino-1,4-anhydro-pentitol and 3-amino-1,5-anhydro-4-deoxy-hexitol with the arabino configuration
- (S)-4-tosyloxymethyl-2-oxazolidinone, required for the synthesis of protected amino alcohol derivatives
- 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine [(S)-HPMPC]
- chiral building block used to construct an epoxyvinyl iodide intermediate in a synthesis of a furanocembrane, a marine natural product
Signal Word
Danger
Hazard Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Carc. 1B - Eye Dam. 1 - Muta. 2 - Repr. 1B - Self-react. C - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
161.6 °F
Flash Point(C)
72 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Chlorobenzilate; 4-Aminobiphenyl; 2-Fluorobiphenyl; N-Nitrosopyrrolidine; 1,2,4,5-Tetrachlorobenzene; 3-Methylcholanthrene; Phenacetin
Chromatograms
suitable for GCsuitable for GCsuitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service