429120
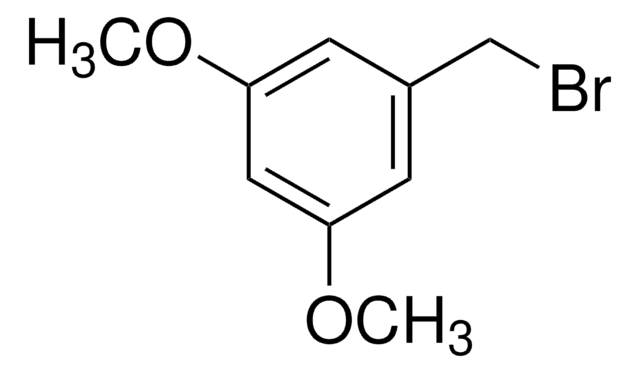
3-Methoxybenzyl bromide
98%
Synonym(s):
1-Bromomethyl-3-methoxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH2Br
CAS Number:
Molecular Weight:
201.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.575 (lit.)
bp
152 °C (lit.)
density
1.436 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
COc1cccc(CBr)c1
InChI
1S/C8H9BrO/c1-10-8-4-2-3-7(5-8)6-9/h2-5H,6H2,1H3
InChI key
ZKSOJQDNSNJIQW-UHFFFAOYSA-N
General description
3-Methoxybenzyl bromide is a benzyl bromide derivative.
Application
3-Methoxybenzyl bromide (1-bromomethyl-3-methoxybenzene) may be used in the diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 6-(3-methoxyphenyl)-hexane-2,4-dione
- N-(3-methoxybenzyl)-N-(1-methyl-1-phenylethyl)-amine
- 2-(3-methoxybenzyl)-3-[(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]-(3S)-2-thionia-bicyclo [2.2.1]- heptane tetrafluoroborate
- 1-(3-methoxybenzyl)-5-(1-methyl-1H-imidazol-5-yl)-1H-1,2,3-triazole
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yang Zhang et al.
The Journal of organic chemistry, 71(12), 4516-4520 (2006-06-06)
A mild protocol for the conversion of beta-ketoesters and beta-diketones to carboxylic acids with use of CAN in CH3CN is described. The method is compatible with a number of functional groups, and can generate carboxylic acids under neutral conditions at
Highly diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives: a new general strategy to (R)-?-arylmethyl-?-butyrolactones.
Pohmakotr M, et al.
Tetrahedron Letters, 45(22), 4315-4318 (2004)
Synthesis of (-)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization.
Clayden J, et al.
Tetrahedron, 58(23), 4727-4733 (2002)
Application of sulfur ylide mediated epoxidations in the asymmetric synthesis of ?-hydroxy-d-lactones. Synthesis of a mevinic acid analogue and (+)-prelactone B.
Aggarwal VK, et al.
Tetrahedron Asymmetry, 60(43), 9725-9733 (2004)
Johan R Johansson et al.
The Journal of organic chemistry, 76(7), 2355-2359 (2011-03-11)
An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the primary alkyl halide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service