405418
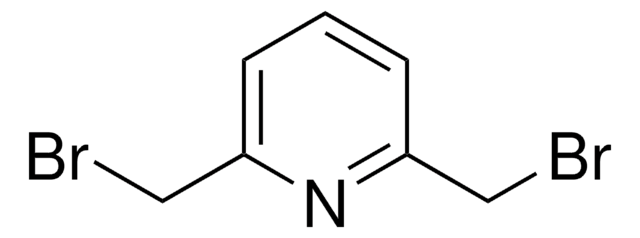
2,6-Bis(chloromethyl)pyridine
99%
Synonym(s):
α,α′-Dichloro-2,6-lutidine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H7Cl2N
CAS Number:
Molecular Weight:
176.04
Beilstein:
116355
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
73-78 °C (lit.)
functional group
chloro
SMILES string
ClCc1cccc(CCl)n1
InChI
1S/C7H7Cl2N/c8-4-6-2-1-3-7(5-9)10-6/h1-3H,4-5H2
InChI key
IWQNFYRJSVJWQA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Bis(chloromethyl)pyridine is a heterocyclic building block for the synthesis of a variety of pyridine derivatives. It coordinates with metal ions through N-atom to form complexes. The conformational flexibility of the bromomethyl arms makes it an ideal choice for the generation of macrocycles. 2,6-bis(chloromethyl)pyridine crystals are monoclinic with space group P21/c. Its synthesis has been reported. The FT-IR and FT-Raman spectra of 2,6-bis(chloromethyl)pyridine (BCMP) have been recorded in the regions 4000-400cm-1 and 3500-100cm-1, respectively.
Application
2,6-Bis(chloromethyl)pyridine may be used in the following studies:
- Synthesis of a sensitive fluorescent chemosensor for Hg2+, composed of two aminonaphthalimide fluorophores and a receptor of 2,6-bis(aminomethyl)pyridine.
- Preparation of carbene pincer ligands, required for the preparation of palladium pincer carbene complex.
- Synthesis of 2-(di-tert-butylphosphinomethyl)-6-diethylaminomethyl)pyridine, PNN ligand.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Iron (II) complexes based on electron-rich, bulky PNN-and PNP-type ligands.
Zhang J, et al.
Inorgorganica Chimica Acta, 359(6), 1955-1960 (2006)
Xiangfeng Guo et al.
Journal of the American Chemical Society, 126(8), 2272-2273 (2004-02-26)
A selective and sensitive fluorescent chemosensor for Hg2+, which was composed of two aminonaphthalimide fluorophores and a receptor of 2,6-bis(aminomethyl)pyridine, was synthesized through the reaction of 2,6-bis(chloromethyl)pyridine and N-[2-(2-hydroxyethoxy)ethyl]-4-piperazino-1,8-naphthalimide. The chemosensor showed an about 17-fold increase in fluorescence quantum yield
Tridentate carbene CCC and CNC pincer palladium (II) complexes: structure, fluxionality, and catalytic activity.
Grundemann S, et al.
Organometallics, 20(25), 5485-5488 (2001)
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 97, 1023-1032 (2012-08-29)
The FT-IR and FT-Raman spectra of 2,6-Bis(chloromethyl)pyridine (BCMP) have been recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively. The total energy calculations of BCMP were tried for the possible rotational isomers. The molecular structure, geometry optimization, vibrational frequencies
An efficient synthesis of 2,6-bis(chloromethyl)pyridine and a [5.5](2,6) pyridinophane disulfite.
Rezzonico B and Grignon-Dubois M.
J. Chem. Res. Synop., 4, 142-143 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service