337331
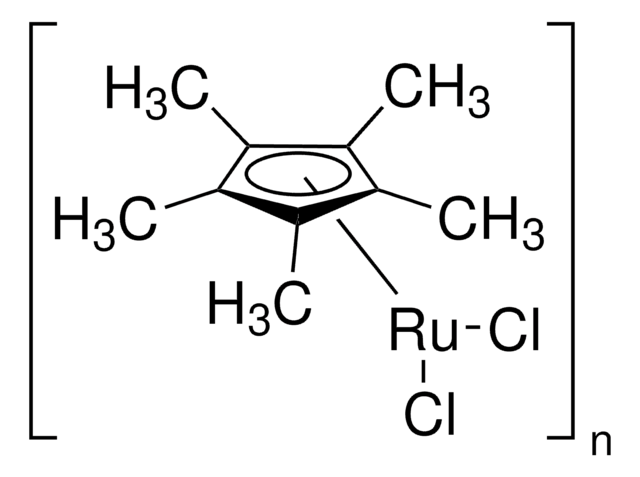
Dichloro(1,5-cyclooctadiene)ruthenium(II), polymer
95%
Synonym(s):
(1,5-Cyclooctadiene)ruthenium(II) chloride, Ruthenium(II) chloride 1,5-cyclooctadiene complex
About This Item
Recommended Products
Assay
95%
reaction suitability
core: ruthenium
reaction type: solution phase peptide synthesis
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
, Aligned
InChI
1S/C8H12.2ClH.Ru/c1-2-4-6-8-7-5-3-1;;;/h1-2,7-8H,3-6H2;2*1H;/q;;;+2/p-2
InChI key
DMRVBCXRFYZCPR-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
Amide Synthesis from Alcohols and Amines by the Extrusion of Dihydrogen
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We are proud to offer a number of products used in catalytic amidation technology.
Protocols
Acetylene chemistry has been and remains an important constituent element of molecular sciences. Its potential and widespread applications extend from organic synthesis through materials science to bioorganic chemistry. Some examples are enediynes (DNA-cleaving agents), ‘click chemistry’ tools and building blocks. Consequently, it triggers a demand for efficient syntheses of alkynes.
Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service