All Photos(2)
About This Item
Linear Formula:
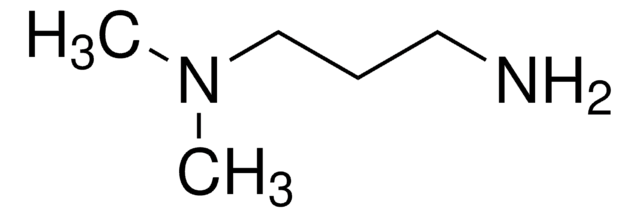
CH3CH2CH2NH(CH2)3NH2
CAS Number:
Molecular Weight:
116.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.4451 (lit.)
bp
169 °C (lit.)
density
0.841 g/mL at 25 °C (lit.)
SMILES string
CCCNCCCN
InChI
1S/C6H16N2/c1-2-5-8-6-3-4-7/h8H,2-7H2,1H3
InChI key
OWKYZAGJTTTXOK-UHFFFAOYSA-N
Related Categories
Application
N-Propyl-1,3-propanediamine was used in the synthesis of 1-benzotriazolylmethyl-3-propylhexahydropyrimidine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alan R Katritzky et al.
The Journal of organic chemistry, 67(9), 3115-3117 (2002-04-27)
1-Benzotriazolylmethyl-3-propylhexahydropyrimidine (1) and 1,3-bis(1H-1,2,3-benzotriazol-1-ylmethyl)-1,2,3,4-tetrahydroquinazoline (3) were readily prepared by reactions of N-propyl-1,3-propanediamine or 2-aminobenzylamine with benzotriazole and formaldehyde, respectively. Intermediate 1 reacted with alkyl and aryl Grignard reagents to produce N,N'-unsymmetrically substituted hexahydropyrimidines 2a,b in 90 and 92% yields, respectively.
Ahmed Salman et al.
Genes, 11(10) (2020-10-04)
In this study, we seek to exclude other pathophysiological mechanisms by which Frmd7 knock-down may cause Idiopathic Infantile Nystagmus (IIN) using the Frmd7.tm1a and Frmd7.tm1b murine models. We used a combination of genetic, histological and visual function techniques to characterize
C M Maragos et al.
Cancer research, 53(3), 564-568 (1993-02-01)
Cell-mediated antitumor effects have, in part, been attributed to the production of NO. Compounds which generate NO might, therefore, be useful in attenuating the growth of tumor cells. Six nitric oxide/nucleophile adducts that release NO spontaneously in solution were tested
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service