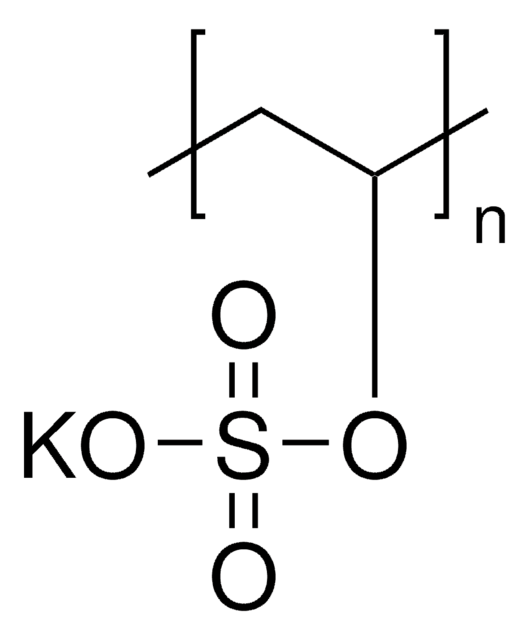
278424
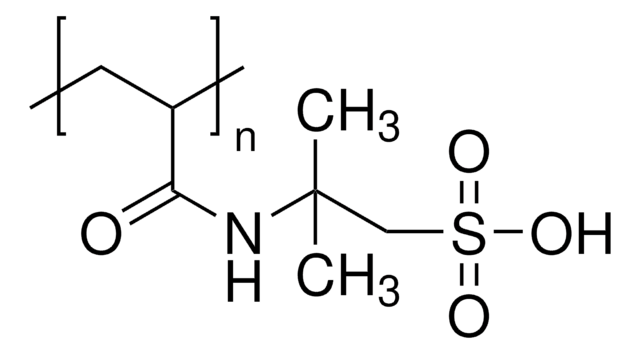
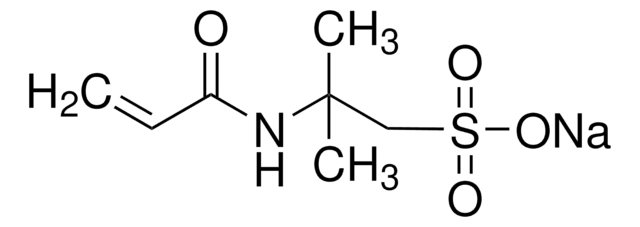
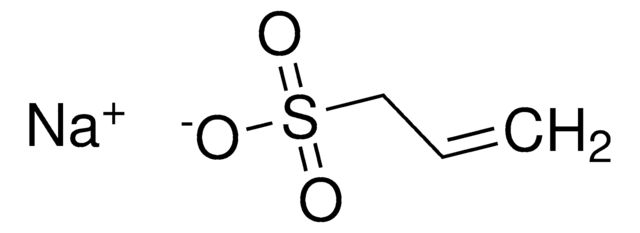
Poly(vinylsulfonic acid, sodium salt) solution
30-40 wt. % in H2O, technical grade
Synonym(s):
PVSA
About This Item
Recommended Products
grade
technical grade
Quality Level
form
liquid
concentration
30-40 wt. % in H2O
refractive index
n20/D 1.389
density
1.267 g/mL at 25 °C
SMILES string
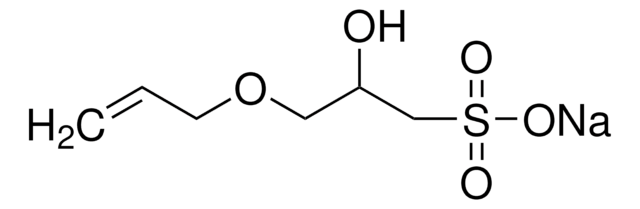
[Na]OS(=O)(=O)C=C
InChI
1S/C2H4O3S.Na/c1-2-6(3,4)5;/h2H,1H2,(H,3,4,5);/q;+1/p-1
InChI key
BWYYYTVSBPRQCN-UHFFFAOYSA-M
Application
- In the preparation of superabsorbent semi-IPN (interpenetrating polymer network) hydrogel.
- As a solid electrolyte for proton conduction in the fabrication of an all-solid-supercapacitor.
- As a crystallization controlling agent in the preparation of high-quality crystals of porous coordination polymers(CP). PVSA regulates not only the size and structure of the crystals but also their preference orientation, resulting in CP channel alignment in the bulk powdery state.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Recently, layer-by-layer (LbL) assembly has emerged as a versatile, gentle and, simple method for immobilization of functional molecules in an easily controllable thin film morphology.1,2 In this short review, we introduce recent advances in functional systems fabricated by using the mild, yet adaptable LbL technique.
We present an article that discusses two applications in particular; first, using these layers as polyelectrolyte membranes to control permeability.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service