All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O
CAS Number:
Molecular Weight:
122.12
Beilstein:
109630
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
76-78 °C (lit.)
functional group
ketone
SMILES string
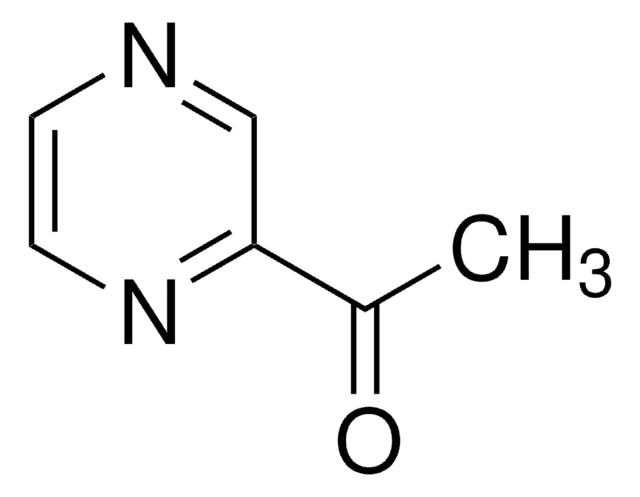
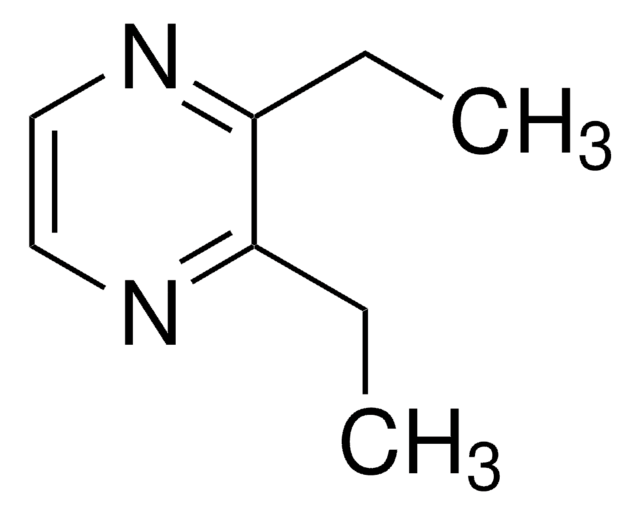
CC(=O)c1cnccn1
InChI
1S/C6H6N2O/c1-5(9)6-4-7-2-3-8-6/h2-4H,1H3
InChI key
DBZAKQWXICEWNW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Acetylpyrazine has been used in synthesis of:
- 7-heteroaryl-pyrazolo[1,5-a]pyrimidine-3-carboxamides
- copper (II) complexes with di-imine ligands
- N(4) substituted thiosemicarbazones
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Spectroscopic characterization of schiff base-copper complexes immobilized in smectite clays.
Dias PM, et al.
Quimica nova, 33(10), 2135-2142 (2010)
Cobalt (III) complexes of formyl-and acetylpyrazine N (4)-substituted thiosemicarbazones.
West DX, et al.
Transition Met. Chem. (London), 22(5), 447-452 (1997)
Sizana Ahmetaj et al.
Molecular diversity, 17(4), 731-743 (2013-08-27)
A simple and practical four-step protocol for the parallel synthesis of 7-heteroaryl-pyrazolo[1,5-[Formula: see text]]pyrimidine-3-carboxamides was developed. The synthesis starts with transformation of commercially available 2-acetylpyridine and acetylpyrazine with [Formula: see text] [Formula: see text]-dimethylformamide dimethylacetal into the corresponding [Formula: see
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service