All Photos(1)
About This Item
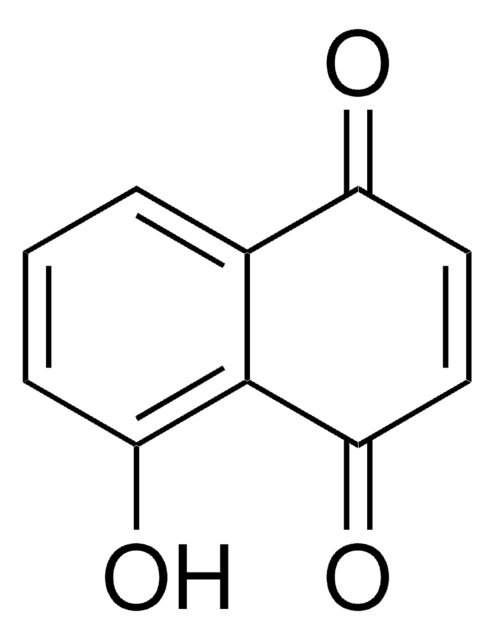
Linear Formula:
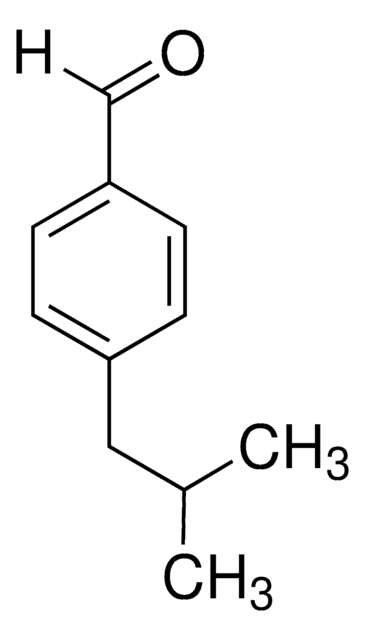
CH3(CH2)3OC6H4CHO
CAS Number:
Molecular Weight:
178.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.539 (lit.)
bp
285 °C (lit.)
density
1.031 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
CCCCOc1ccc(C=O)cc1
InChI
1S/C11H14O2/c1-2-3-8-13-11-6-4-10(9-12)5-7-11/h4-7,9H,2-3,8H2,1H3
InChI key
XHWMNHADTZZHGI-UHFFFAOYSA-N
Related Categories
General description
Kinetic constant for the inhibition of the diphenolase activity of mushroom tyrosinase by 4-butoxybenzaldehyde has been evaluated.
Application
4-Butoxybenzaldehyde has been used in the synthesis of:
- 6-amino-4-(4-butoxyphenyl)-3,5-dicyanopyridine-2(1H)-thione
- 16-(p-butoxybenzylidene)androsta-1,4-diene-3,17-dione via condensation reaction with androsta-1,4-diene-3,17-dione
Legal Information
Darkens in storage with no loss in purity
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>235.4 °F - closed cup
Flash Point(C)
> 113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
16-(p-Butoxybenzylidene) androsta-1, 4-diene-3, 17-dione.
Ogawa K, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(7), 1359-1361 (1992)
Michael reaction in synthesis of 6-amino-4-(4-butoxyphenyl)-3, 5-dicyanopyridine-2 (1H)-thione.
Dyachenko VD and Litvinov VP.
Chemistry of Heterocyclic Compounds, 34(2), 188-194 (1998)
Dalila Rocco et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-12)
The preparation of 24-functionalized 12,22:26,32-terpyridines (4'-functionalized 3,2:6',3''-terpyridines) by the reaction of three 4-alkoxybenzaldehydes with 3-acetylpyridine and ammonia was investigated; under identical reaction conditions, two (R = nC4H9, C2H5) gave the expected products whereas a third (R = nC3H7) gave only
M Jiménez et al.
Journal of agricultural and food chemistry, 49(8), 4060-4063 (2001-08-22)
A kinetic study of the inhibition of mushroom tyrosinase by 4-substituted benzaldehydes showed that these compounds behave as classical competitive inhibitors, inhibiting the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) by mushroom tyrosinase (o-diphenolase activity). The kinetic parameter (K(I)) characterizing this inhibition was
Naoko Ueno et al.
Langmuir : the ACS journal of surfaces and colloids, 33(22), 5393-5397 (2017-05-16)
We evaluated the speed profile of self-propelled underwater oil droplets comprising a hydrophobic aldehyde derivative in terms of their diameter and the surrounding surfactant concentration using a microfluidic device. We found that the speed of the oil droplets is dependent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service