All Photos(1)
About This Item
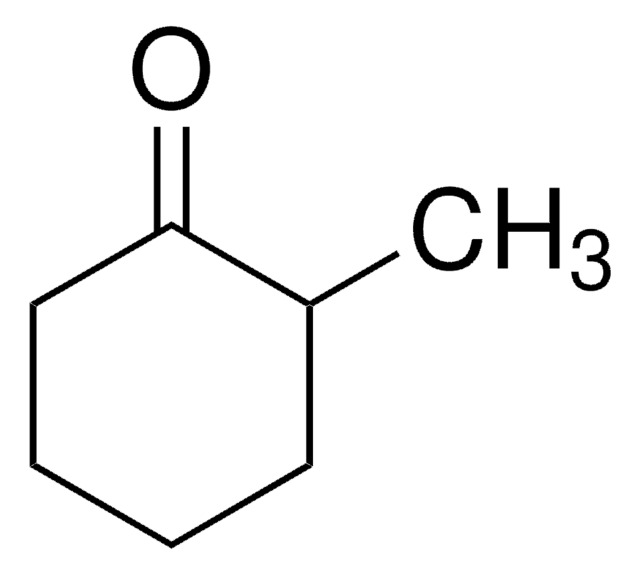
Linear Formula:
CH3OC6H9(=O)
CAS Number:
Molecular Weight:
128.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.455 (lit.)
bp
185 °C/750 mmHg (lit.)
density
1.02 g/mL at 25 °C (lit.)
functional group
ether
ketone
SMILES string
COC1CCCCC1=O
InChI
1S/C7H12O2/c1-9-7-5-3-2-4-6(7)8/h7H,2-5H2,1H3
InChI key
JYJURPHZXCLFDX-UHFFFAOYSA-N
General description
The conformational equilibrium of 2-methoxycyclohexanone was studied by infrared spectroscopy.
Application
2-Methoxycyclohexanone was used in the synthesis of 2-(carbomethoxy)cyclohex-2-en-1-one.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M P Freitas et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 59(6), 1177-1182 (2003-03-28)
The conformational equilibrium of 2-methoxycyclohexanone has been analyzed through infrared spectroscopy and theoretical calculations. These calculations indicate that six conformations may be present in the vapor phase, due to the rotation of the methoxy group around the O-C(2) bond, leading
O Hamed et al.
The Journal of organic chemistry, 66(1), 180-185 (2001-06-30)
Unsubstituted or alkyl-substituted cyclic ketones react with PdCl2 in methanol under a CO atmosphere to give mainly acyclic diesters along with some acyclic chloro-substituted monoesters. The monosubstituted cyclic ketones, 2-hydroxy- and 2-methoxycyclohexanone, do not give ring cleavage but rather produce
Qinglei Meng et al.
Nature communications, 8, 14190-14190 (2017-02-01)
Cyclohexanone and its derivatives are very important chemicals, which are currently produced mainly by oxidation of cyclohexane or alkylcyclohexane, hydrogenation of phenols, and alkylation of cyclohexanone. Here we report that bromide salt-modified Pd/C in H
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service