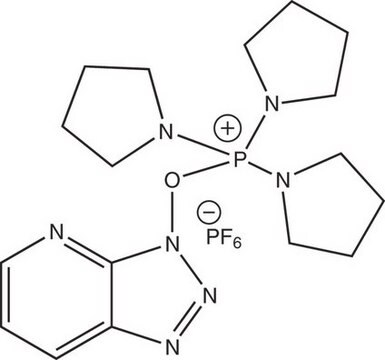
226084
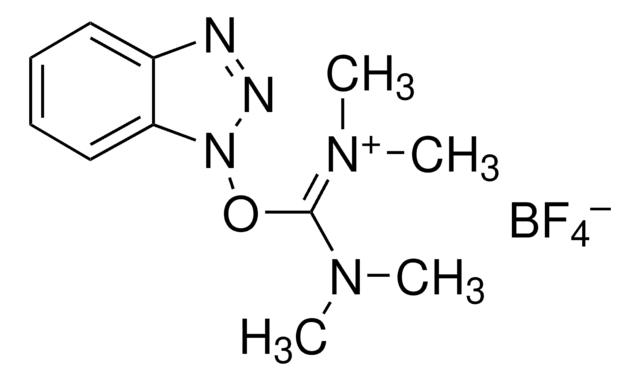
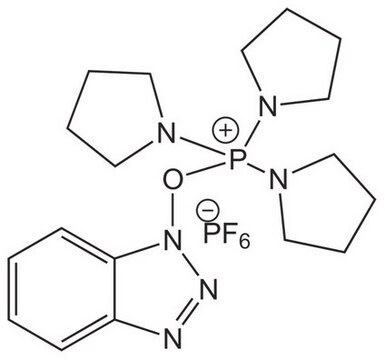
(Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate
97%, for peptide synthesis
Synonym(s):
BOP, BOP Reagent, Castros reagent
About This Item
Recommended Products
product name
(Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, 97%
Quality Level
Assay
97%
form
powder
reaction suitability
reaction type: Coupling Reactions
mp
>130 °C (dec.) (lit.)
application(s)
peptide synthesis
functional group
amine
storage temp.
2-8°C
SMILES string
F[P-](F)(F)(F)(F)F.CN(C)[P+](On1nnc2ccccc12)(N(C)C)N(C)C
InChI
1S/C12H22N6OP.F6P/c1-15(2)20(16(3)4,17(5)6)19-18-12-10-8-7-9-11(12)13-14-18;1-7(2,3,4,5)6/h7-10H,1-6H3;/q+1;-1
InChI key
MGEVGECQZUIPSV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Peptide coupling
Synthesis of esters
Esterification of carboxylic acids
Plasmid CAN-encapsulating liposomes
Synthesis of magnolamide for antioxidative activity
Catalyst for preparation of 9-acridinecaroboxamide derivative
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)